air (O2+3.76N2) supplied at the same pressure and temperature. Assume that there is 10% ess air than stoichiometric conditions. Write the chemical reaction equation for the combustion of propane with 10% excess air (a=1.1*a,toich)- If the combustion is not complete, the products may contain CO2, H20, CO, H2, O2 and N2. Rewrite the chemical reaction equation you found in part 1 to include these products. Also, express the relationships between the moles of product species using atom balances. Calculate the adiabatic flame temperature and equilibrium composition. To minimize the iterative steps, make a first guess of 2200 K and a second guess of 2750K. Do this problem by hand, but you may use combustion codes to check your work.
air (O2+3.76N2) supplied at the same pressure and temperature. Assume that there is 10% ess air than stoichiometric conditions. Write the chemical reaction equation for the combustion of propane with 10% excess air (a=1.1*a,toich)- If the combustion is not complete, the products may contain CO2, H20, CO, H2, O2 and N2. Rewrite the chemical reaction equation you found in part 1 to include these products. Also, express the relationships between the moles of product species using atom balances. Calculate the adiabatic flame temperature and equilibrium composition. To minimize the iterative steps, make a first guess of 2200 K and a second guess of 2750K. Do this problem by hand, but you may use combustion codes to check your work.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.32E: Many compressed gases come in large,heavy metal cylindersthat are so heavy that they need a special...
Related questions
Question
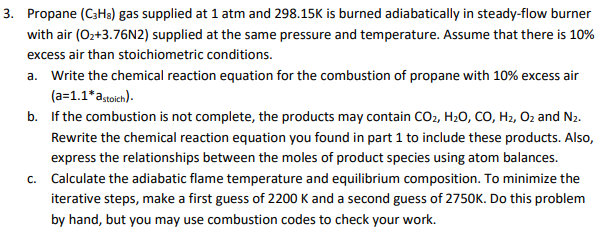
Transcribed Image Text:3. Propane (C3Hs) gas supplied at 1 atm and 298.15K is burned adiabatically in steady-flow burner
with air (O2+3.76N2) supplied at the same pressure and temperature. Assume that there is 10%
excess air than stoichiometric conditions.
a. Write the chemical reaction equation for the combustion of propane with 10% excess air
(a=1.1*a,toich).
b. If the combustion is not complete, the products may contain CO2, H20, CO, H2, Oz and N2.
Rewrite the chemical reaction equation you found in part 1 to include these products. Also,
express the relationships between the moles of product species using atom balances.
c. Calculate the adiabatic flame temperature and equilibrium composition. To minimize the
iterative steps, make a first guess of 2200 K and a second guess of 2750K. Do this problem
by hand, but you may use combustion codes to check your work.
Expert Solution
Step 1


Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,