Table B.2 of App. B provides parameters for an equation that gives Psat as a function of T for a number of pure compounds. For one of them, determine the heat of vaporization at its normal boiling point by application of Eq. (4.12), the Clapeyron equation. Evaluate dPsat/dT from the given vapor-pressure equation, and use generalized correlations from Chapter 3 to estimate AV. Compare the computed value with the value of AHn listed in Table B.2. Note that normal boiling points are listed in the last column of Table B.2. Equation (4.12) dpsat ДН - ТАУ- đT Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species B In Psat /kPa = A –- 1/°C + C Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (1,) Parameters for Antoine Eqn. Temp. Range AH, Name Formula At °C kJ/mol t„°C C3H¿O 14.3145 2756.22 228.060 -26–77 C2H4O2 15.0717 3580.80 224.650 CH3N 14.8950 3413.10 250.523 -27–81 13.7819 2726.81 217.572 13 8254 2181 .79. 248 870 Acetone 29.10 56.2 Acetic acid 24–142 23.70 117.9 Acetonitrile* 30.19 81.6 Benzene C¿H6 6-104 30.72 80.0 ion Butane 21 30 Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (f,) Parameters for Antoine Eqn. Temp. Range AH, At в с Name Formula °C kJ/mol t„°C C;HO 14.3145 2756.22 228.060 -26–77 CH,O2 15.0717 3580.80 224.650 C,H3N C;H6 СаНо СдНо C4H100 15.3144 3212.43 182.739 C4H190 15.1989 3026.03 186.500 C4H100 14.6047 2740.95 166.670 C4H100 14.8445 2658.29 177.650 Acetone 29.10 56.2 Acetic acid 24–142 23.70 117.9 Acetonitrile* 14.8950 3413.10 250.523 -27–81 30.19 81.6 Benzene 13.7819 2726.81 217.572 6-104 30.72 80.0 iso-Butane 13.8254 2181.79 248.870 -83–7 21.30 -11.9 n-Butane 1-Butanol 2-Butanol* 13.6608 2154.70 238.789 -73–19 22.44 -0.5 37–138 43.29 117.6 25–120 40.75 99.5 iso-Butanol 30–128 41.82 107.8 tert-Butanol 10–101 39.07 82.3 14.0572 2914.23 232.148 -14–101 76.6 Carbon tetrachloride CCI4 Chlorobenzene 29.82 C;H;Cl 13.8635 3174.78 211.700 CạH,Cl 13.7965 2723.73 218.265 -17–79 CHCI3 C;H12 C3H10 C10H22 13.9748 3442.76 193.858 CH2C2 13.9891 2463.93 223.240 -38–60 C4H190 14.0735 2511.29 231.200 -43-55 C4H&O2 15.0967 3579.78 240.337 C20H42 14.4575 4680.46 132.100 208-379 CHO 16.8958 3795.17 230.918 C3H10 CH,O2 15.7567 4187.46 178.650 C;H16 C;H14 CH40 C3H¿O2 14.2456 2662.78 219.690 -23–78 29–159 35.19 131.7 1-Chlorobutane 30.39 78.5 Chloroform 13.7324 2548.74 218.552 -23–84 29.24 61.1 13.6568 2723.44 220.618 29.97 Cyclohexane Cyclopentane n-Decane 9–105 80.7 13.9727 2653.90 234.510 -35–71 27.30 49.2 65-203 38.75 174.1 39.7 34.4 Dichloromethane 28.06 26.52 Diethyl ether 1,4-Dioxane 20–105 34.16 101.3 n-Eicosane Ethanol 57.49 343.6 38.56 35.57 136.2 3–96 78.2 13.9726 3259.93 212.300 33–163 Ethylbenzene Ethylene glycol* n-Heptane n-Hexane 100–222 50.73 197.3 13.8622 2910.26 216.432 13.8193 2696.04 224.317 -19–92 4-123 31.77 98.4 28.85 68.7 Methanol 16.5785 3638.27 239.500 -11–83 35.21 64.7 Methyl acetate Methyl ethyl ketone CH3O Nitromethane* 30.32 56.9 14.1334 2838.24 218.690 -8–103 31.30 79.6 CH3NO, 14.7513 3331.70 227.600 С,Ндо C3H18 C3H18 C;H12 C;HO 14.4387 3507.80 175.400 CэHао C;HgO 16.6796 3640.20 219.610 56–146 33.99 101.2 n-Nonane 13.9854 3311.19 202.694 46–178 36.91 150.8 iso-Octane n-Octane 13.6703 2896.31 220.767 2–125 30.79 99.2 13.9346 3123.13 209.635 13.7667 2451.88 232.014 -45–58 26–152 34.41 125.6 25.79 n-Pentane 36.0 Phenol 46.18 181.8 97.2 80–208 16.1154 3483.67 205.807 1-Propanol 2-Propanol 20–116 41.44 8-100 39.85 82.2 Parameters for Antoine Eqn. Temp. Range AH, Name Formula At B c °C kJ/mol i„°C 33.18 110.6 C;H3 Но C3H10 C3H10 C3H10 Toluene 13.9320 3056.96 217.625 13–136 Water 16.3872 3885.70 230.170 0-200 40.66 100.0 o-Xylene m-Xylene p-Xylene 14.0415 3358.79 212.041 40-172 36.24 144.4 35–166 35.66 139.1 14.1387 3381.81 216.120 14.0579 3331.45 214.627 35–166 35.67 138.3
Table B.2 of App. B provides parameters for an equation that gives Psat as a function of T for a number of pure compounds. For one of them, determine the heat of vaporization at its normal boiling point by application of Eq. (4.12), the Clapeyron equation. Evaluate dPsat/dT from the given vapor-pressure equation, and use generalized correlations from Chapter 3 to estimate AV. Compare the computed value with the value of AHn listed in Table B.2. Note that normal boiling points are listed in the last column of Table B.2. Equation (4.12) dpsat ДН - ТАУ- đT Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species B In Psat /kPa = A –- 1/°C + C Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (1,) Parameters for Antoine Eqn. Temp. Range AH, Name Formula At °C kJ/mol t„°C C3H¿O 14.3145 2756.22 228.060 -26–77 C2H4O2 15.0717 3580.80 224.650 CH3N 14.8950 3413.10 250.523 -27–81 13.7819 2726.81 217.572 13 8254 2181 .79. 248 870 Acetone 29.10 56.2 Acetic acid 24–142 23.70 117.9 Acetonitrile* 30.19 81.6 Benzene C¿H6 6-104 30.72 80.0 ion Butane 21 30 Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (f,) Parameters for Antoine Eqn. Temp. Range AH, At в с Name Formula °C kJ/mol t„°C C;HO 14.3145 2756.22 228.060 -26–77 CH,O2 15.0717 3580.80 224.650 C,H3N C;H6 СаНо СдНо C4H100 15.3144 3212.43 182.739 C4H190 15.1989 3026.03 186.500 C4H100 14.6047 2740.95 166.670 C4H100 14.8445 2658.29 177.650 Acetone 29.10 56.2 Acetic acid 24–142 23.70 117.9 Acetonitrile* 14.8950 3413.10 250.523 -27–81 30.19 81.6 Benzene 13.7819 2726.81 217.572 6-104 30.72 80.0 iso-Butane 13.8254 2181.79 248.870 -83–7 21.30 -11.9 n-Butane 1-Butanol 2-Butanol* 13.6608 2154.70 238.789 -73–19 22.44 -0.5 37–138 43.29 117.6 25–120 40.75 99.5 iso-Butanol 30–128 41.82 107.8 tert-Butanol 10–101 39.07 82.3 14.0572 2914.23 232.148 -14–101 76.6 Carbon tetrachloride CCI4 Chlorobenzene 29.82 C;H;Cl 13.8635 3174.78 211.700 CạH,Cl 13.7965 2723.73 218.265 -17–79 CHCI3 C;H12 C3H10 C10H22 13.9748 3442.76 193.858 CH2C2 13.9891 2463.93 223.240 -38–60 C4H190 14.0735 2511.29 231.200 -43-55 C4H&O2 15.0967 3579.78 240.337 C20H42 14.4575 4680.46 132.100 208-379 CHO 16.8958 3795.17 230.918 C3H10 CH,O2 15.7567 4187.46 178.650 C;H16 C;H14 CH40 C3H¿O2 14.2456 2662.78 219.690 -23–78 29–159 35.19 131.7 1-Chlorobutane 30.39 78.5 Chloroform 13.7324 2548.74 218.552 -23–84 29.24 61.1 13.6568 2723.44 220.618 29.97 Cyclohexane Cyclopentane n-Decane 9–105 80.7 13.9727 2653.90 234.510 -35–71 27.30 49.2 65-203 38.75 174.1 39.7 34.4 Dichloromethane 28.06 26.52 Diethyl ether 1,4-Dioxane 20–105 34.16 101.3 n-Eicosane Ethanol 57.49 343.6 38.56 35.57 136.2 3–96 78.2 13.9726 3259.93 212.300 33–163 Ethylbenzene Ethylene glycol* n-Heptane n-Hexane 100–222 50.73 197.3 13.8622 2910.26 216.432 13.8193 2696.04 224.317 -19–92 4-123 31.77 98.4 28.85 68.7 Methanol 16.5785 3638.27 239.500 -11–83 35.21 64.7 Methyl acetate Methyl ethyl ketone CH3O Nitromethane* 30.32 56.9 14.1334 2838.24 218.690 -8–103 31.30 79.6 CH3NO, 14.7513 3331.70 227.600 С,Ндо C3H18 C3H18 C;H12 C;HO 14.4387 3507.80 175.400 CэHао C;HgO 16.6796 3640.20 219.610 56–146 33.99 101.2 n-Nonane 13.9854 3311.19 202.694 46–178 36.91 150.8 iso-Octane n-Octane 13.6703 2896.31 220.767 2–125 30.79 99.2 13.9346 3123.13 209.635 13.7667 2451.88 232.014 -45–58 26–152 34.41 125.6 25.79 n-Pentane 36.0 Phenol 46.18 181.8 97.2 80–208 16.1154 3483.67 205.807 1-Propanol 2-Propanol 20–116 41.44 8-100 39.85 82.2 Parameters for Antoine Eqn. Temp. Range AH, Name Formula At B c °C kJ/mol i„°C 33.18 110.6 C;H3 Но C3H10 C3H10 C3H10 Toluene 13.9320 3056.96 217.625 13–136 Water 16.3872 3885.70 230.170 0-200 40.66 100.0 o-Xylene m-Xylene p-Xylene 14.0415 3358.79 212.041 40-172 36.24 144.4 35–166 35.66 139.1 14.1387 3381.81 216.120 14.0579 3331.45 214.627 35–166 35.67 138.3
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Table B.2 App. B provides parameter for an equation that give Psat as a function of T for a number of pure compounds. For one them determine the heat of vaporization at its normal boiling point by application equation 4.12 the clepeyron equation. Evaluate dPsat/dT form vapor pressure equation an it use generalized Correlations from chapter 3 to estimate delta V compare the computer value with the value delta Hn listed in table B2. Note that normal boiling point are listed in the last column of table B.2
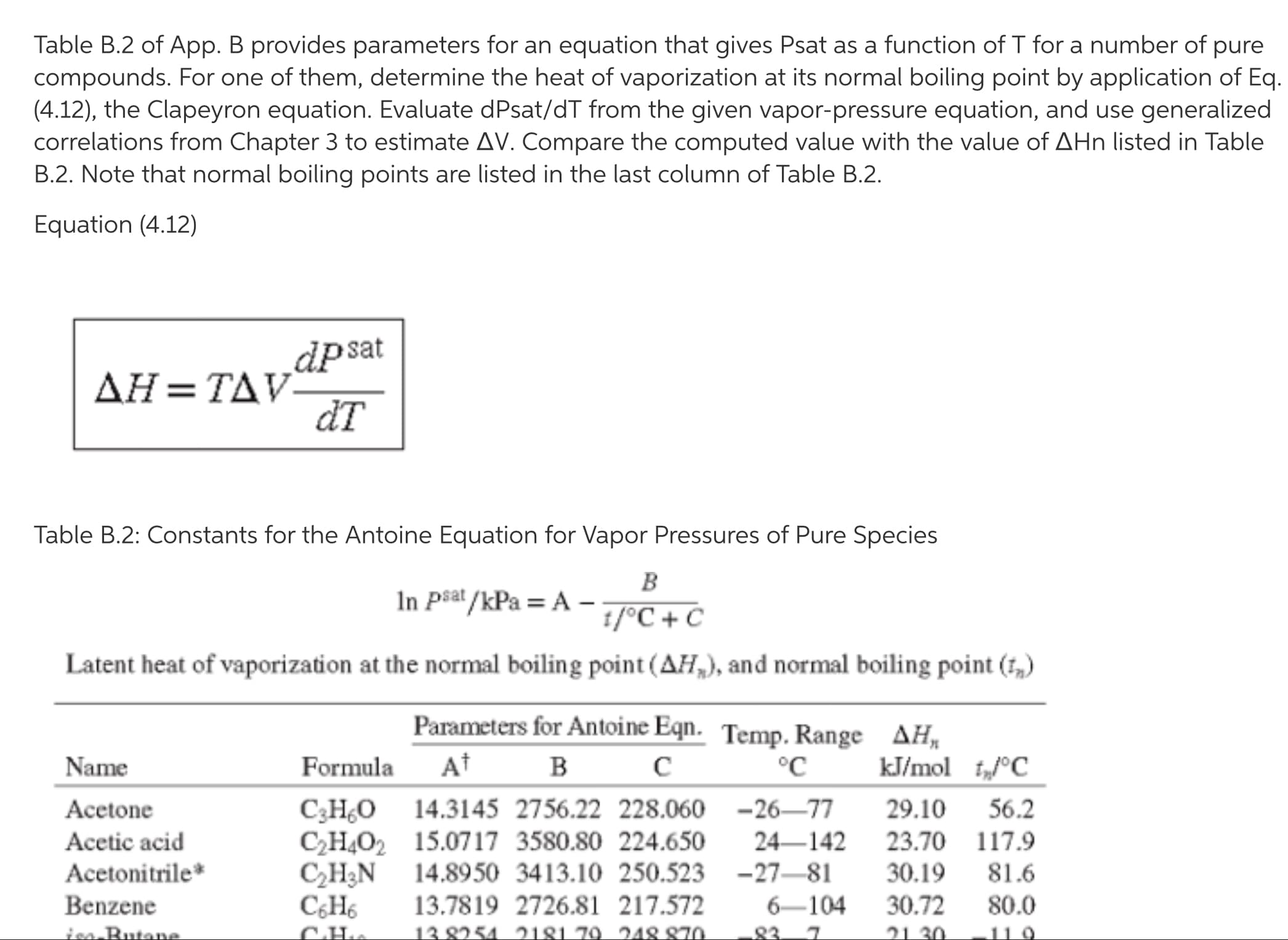
Transcribed Image Text:Table B.2 of App. B provides parameters for an equation that gives Psat as a function of T for a number of pure
compounds. For one of them, determine the heat of vaporization at its normal boiling point by application of Eq.
(4.12), the Clapeyron equation. Evaluate dPsat/dT from the given vapor-pressure equation, and use generalized
correlations from Chapter 3 to estimate AV. Compare the computed value with the value of AHn listed in Table
B.2. Note that normal boiling points are listed in the last column of Table B.2.
Equation (4.12)
dpsat
ДН - ТАУ-
đT
Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species
B
In Psat /kPa = A –-
1/°C + C
Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (1,)
Parameters for Antoine Eqn. Temp. Range AH,
Name
Formula
At
°C
kJ/mol t„°C
C3H¿O 14.3145 2756.22 228.060 -26–77
C2H4O2 15.0717 3580.80 224.650
CH3N 14.8950 3413.10 250.523 -27–81
13.7819 2726.81 217.572
13 8254 2181 .79. 248 870
Acetone
29.10
56.2
Acetic acid
24–142
23.70 117.9
Acetonitrile*
30.19
81.6
Benzene
C¿H6
6-104
30.72
80.0
ion Butane
21 30
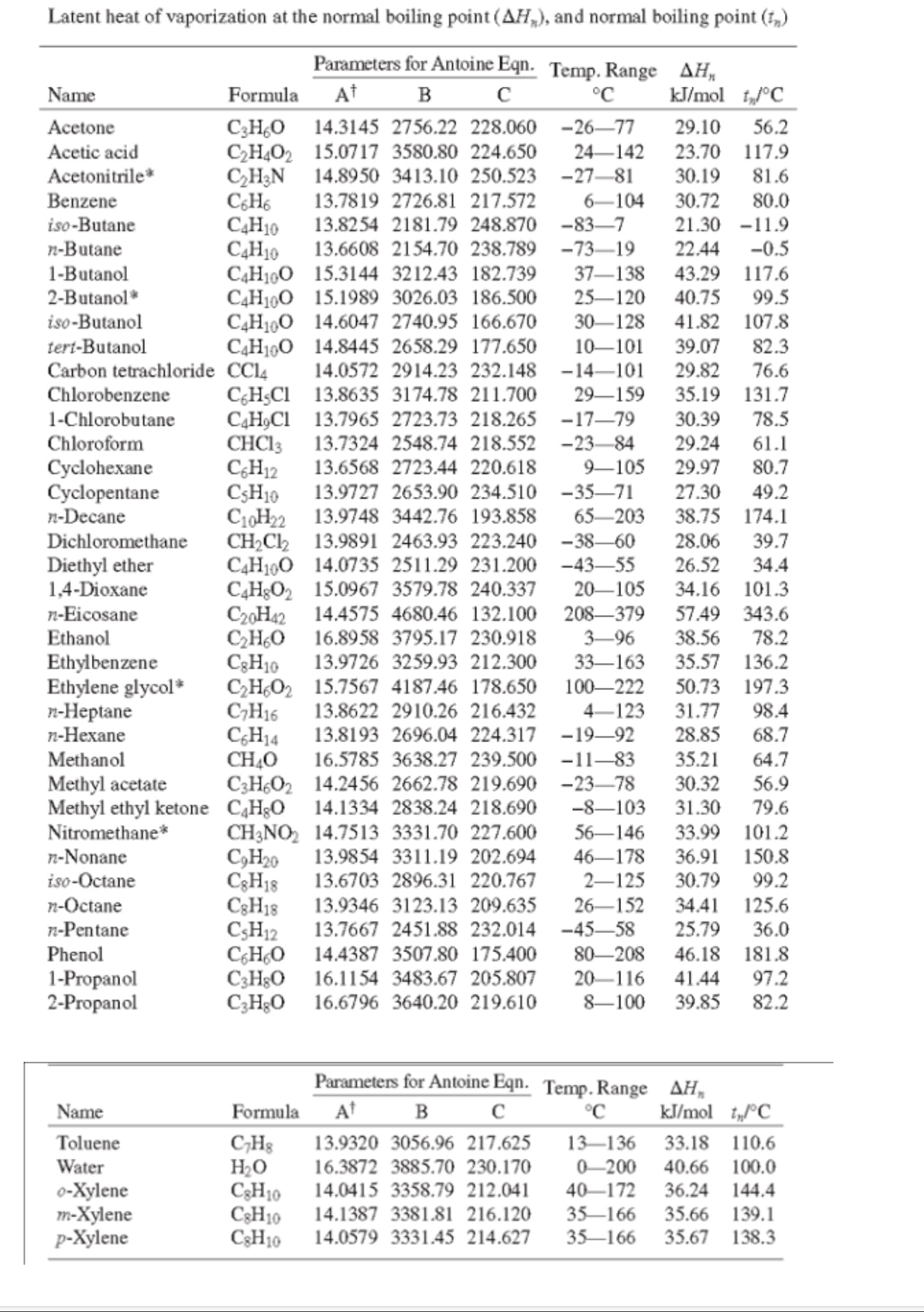
Transcribed Image Text:Latent heat of vaporization at the normal boiling point (AH,), and normal boiling point (f,)
Parameters for Antoine Eqn. Temp. Range AH,
At
в с
Name
Formula
°C
kJ/mol t„°C
C;HO 14.3145 2756.22 228.060 -26–77
CH,O2 15.0717 3580.80 224.650
C,H3N
C;H6
СаНо
СдНо
C4H100 15.3144 3212.43 182.739
C4H190 15.1989 3026.03 186.500
C4H100 14.6047 2740.95 166.670
C4H100 14.8445 2658.29 177.650
Acetone
29.10
56.2
Acetic acid
24–142
23.70 117.9
Acetonitrile*
14.8950 3413.10 250.523 -27–81
30.19
81.6
Benzene
13.7819 2726.81 217.572
6-104
30.72
80.0
iso-Butane
13.8254 2181.79 248.870 -83–7
21.30 -11.9
n-Butane
1-Butanol
2-Butanol*
13.6608 2154.70 238.789 -73–19
22.44 -0.5
37–138
43.29 117.6
25–120
40.75 99.5
iso-Butanol
30–128
41.82 107.8
tert-Butanol
10–101
39.07
82.3
14.0572 2914.23 232.148 -14–101
76.6
Carbon tetrachloride CCI4
Chlorobenzene
29.82
C;H;Cl 13.8635 3174.78 211.700
CạH,Cl 13.7965 2723.73 218.265 -17–79
CHCI3
C;H12
C3H10
C10H22 13.9748 3442.76 193.858
CH2C2 13.9891 2463.93 223.240 -38–60
C4H190 14.0735 2511.29 231.200 -43-55
C4H&O2 15.0967 3579.78 240.337
C20H42 14.4575 4680.46 132.100 208-379
CHO 16.8958 3795.17 230.918
C3H10
CH,O2 15.7567 4187.46 178.650
C;H16
C;H14
CH40
C3H¿O2 14.2456 2662.78 219.690 -23–78
29–159
35.19 131.7
1-Chlorobutane
30.39
78.5
Chloroform
13.7324 2548.74 218.552 -23–84
29.24
61.1
13.6568 2723.44 220.618
29.97
Cyclohexane
Cyclopentane
n-Decane
9–105
80.7
13.9727 2653.90 234.510 -35–71
27.30
49.2
65-203
38.75 174.1
39.7
34.4
Dichloromethane
28.06
26.52
Diethyl ether
1,4-Dioxane
20–105
34.16 101.3
n-Eicosane
Ethanol
57.49 343.6
38.56
35.57 136.2
3–96
78.2
13.9726 3259.93 212.300
33–163
Ethylbenzene
Ethylene glycol*
n-Heptane
n-Hexane
100–222
50.73 197.3
13.8622 2910.26 216.432
13.8193 2696.04 224.317 -19–92
4-123
31.77
98.4
28.85
68.7
Methanol
16.5785 3638.27 239.500 -11–83
35.21
64.7
Methyl acetate
Methyl ethyl ketone CH3O
Nitromethane*
30.32
56.9
14.1334 2838.24 218.690
-8–103
31.30
79.6
CH3NO, 14.7513 3331.70 227.600
С,Ндо
C3H18
C3H18
C;H12
C;HO 14.4387 3507.80 175.400
CэHао
C;HgO 16.6796 3640.20 219.610
56–146
33.99 101.2
n-Nonane
13.9854 3311.19 202.694
46–178
36.91 150.8
iso-Octane
n-Octane
13.6703 2896.31 220.767
2–125
30.79
99.2
13.9346 3123.13 209.635
13.7667 2451.88 232.014 -45–58
26–152
34.41 125.6
25.79
n-Pentane
36.0
Phenol
46.18 181.8
97.2
80–208
16.1154 3483.67 205.807
1-Propanol
2-Propanol
20–116
41.44
8-100
39.85
82.2
Parameters for Antoine Eqn. Temp. Range AH,
Name
Formula
At B c
°C
kJ/mol i„°C
33.18 110.6
C;H3
Но
C3H10
C3H10
C3H10
Toluene
13.9320 3056.96 217.625
13–136
Water
16.3872 3885.70 230.170
0-200
40.66 100.0
o-Xylene
m-Xylene
p-Xylene
14.0415 3358.79 212.041
40-172
36.24 144.4
35–166
35.66 139.1
14.1387 3381.81 216.120
14.0579 3331.45 214.627
35–166
35.67 138.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 13 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The