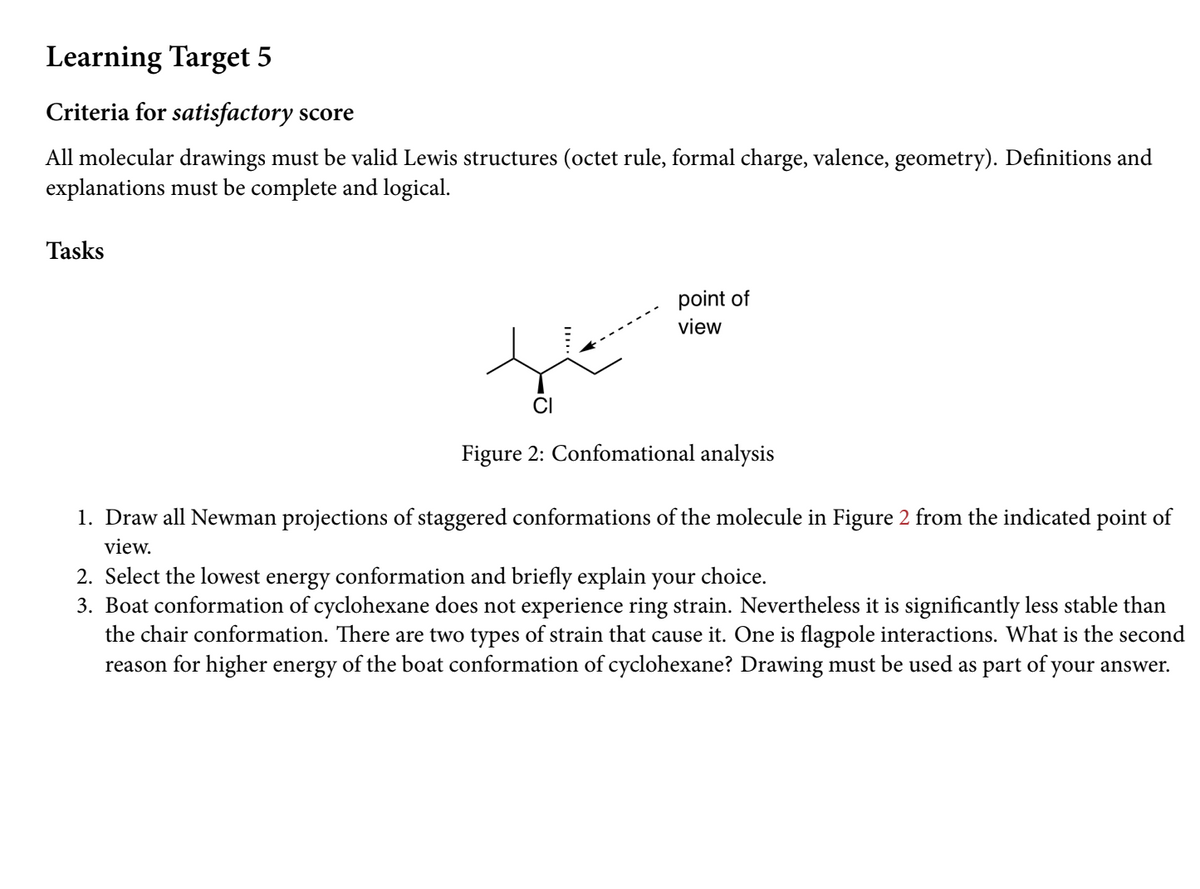
Tasks point of view CI Figure 2: Confomational analysis 1. Draw all Newman projections of staggered conformations of the molecule in Figure 2 from the indicated point of view. 2. Select the lowest energy conformation and briefly explain your choice. 3. Boat conformation of cyclohexane does not experience ring strain. Nevertheless it is significantly less stable than the chair conformation. There are two types of strain that cause it. One is flagpole interactions. What is the second reason for higher energy of the boat conformation of cyclohexane? Drawing must be used as part of your answer.
Tasks point of view CI Figure 2: Confomational analysis 1. Draw all Newman projections of staggered conformations of the molecule in Figure 2 from the indicated point of view. 2. Select the lowest energy conformation and briefly explain your choice. 3. Boat conformation of cyclohexane does not experience ring strain. Nevertheless it is significantly less stable than the chair conformation. There are two types of strain that cause it. One is flagpole interactions. What is the second reason for higher energy of the boat conformation of cyclohexane? Drawing must be used as part of your answer.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter6: Alkanes & Alkenes
Section: Chapter Questions
Problem 10CTQ: Consider the Newman projection below. a. Draw a full Lewis structure of this molecule with...
Related questions
Question
100%
Transcribed Image Text:Learning Target 5
Criteria for satisfactory score
All molecular drawings must be valid Lewis structures (octet rule, formal charge, valence, geometry). Definitions and
explanations must be complete and logical.
Tasks
point of
view
CI
Figure 2: Confomational analysis
1. Draw all Newman projections of staggered conformations of the molecule in Figure 2 from the indicated point of
view.
2. Select the lowest energy conformation and briefly explain your choice.
3. Boat conformation of cyclohexane does not experience ring strain. Nevertheless it is significantly less stable than
the chair conformation. There are two types of strain that cause it. One is flagpole interactions. What is the second
reason for higher energy of the boat conformation of cyclohexane? Drawing must be used as part of your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole