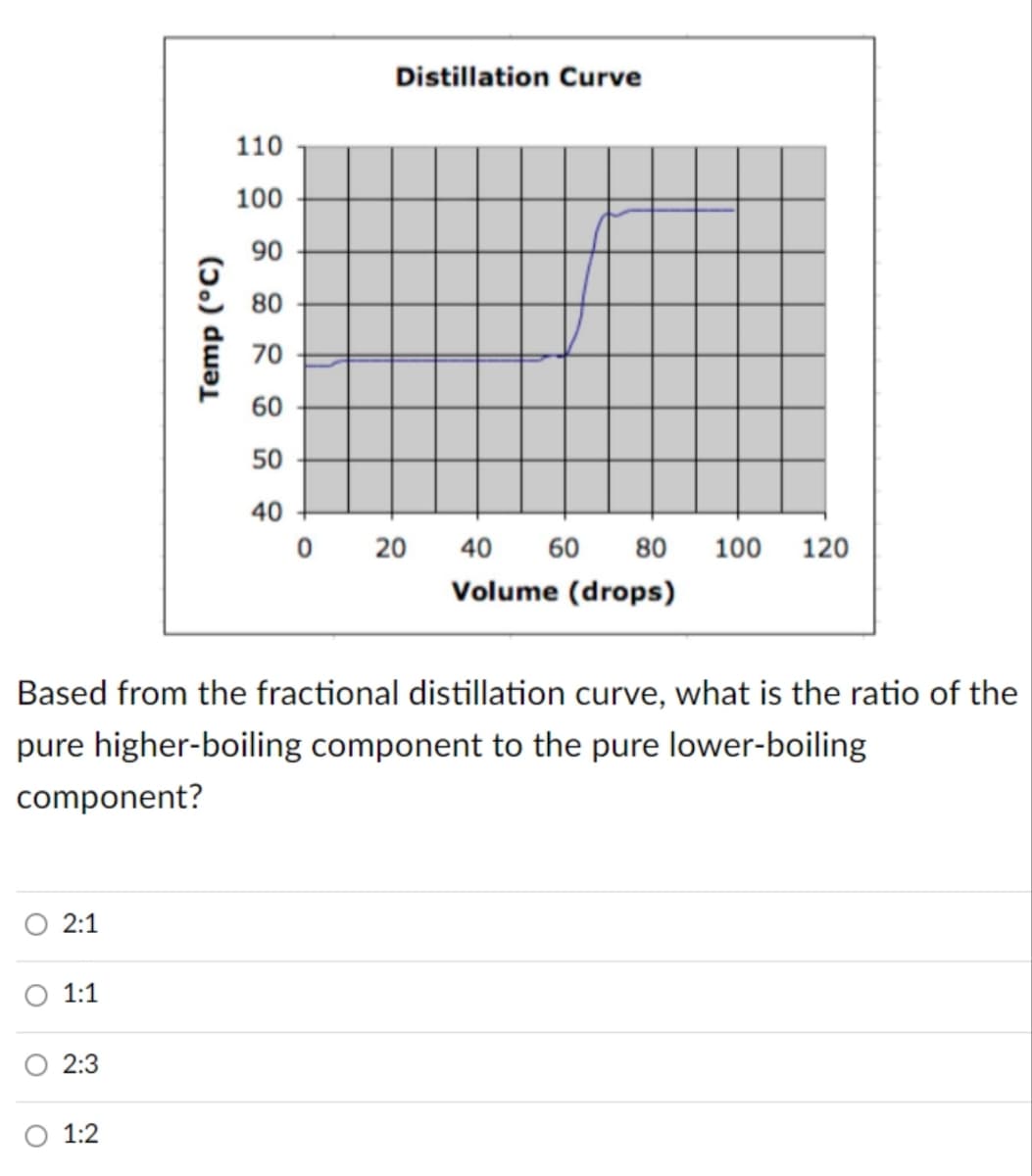
Temp (°C) 110 100 90 80 70 60 50 40 Distillation Curve 0 20 40 60 80 100 120 Volume (drops) Based from the fractional distillation curve, what is the ratio of th pure higher-boiling component to the pure lower-boiling component?
Q: ded for A sample of argon gas occupies a volume of 6.83 L at 42.0°C and 0.960 atm. If it is desired…
A:
Q: A hot air balloon is filled with 635 L of Helium at 22 °C and pressure of 0.934 atm. What is the…
A: Given - Volume= 635 L = V1= V2 T1=22°C = 22 + 273 = 295 K T2=155°C = 155 + 273 = 428 K P1=0.934atm…
Q: Add a methyl substituent at carbon #2, an ethyl substituent at carbon and an isopropyl substituent…
A:
Q: Choose the resonance structure that results from the arrow pushing scheme below: O-H سفه مان 0- نی…
A: Whenever for a molecule we can write two or more Lewis structures which differ in the position of…
Q: Balance the following redox reactions in basic solution CH,CHO(I) + Cu(s) CH₂COO (aq) + Cu₂O(s)…
A: According to the bartleby guidelines for the multipart questions I can solve only first three…
Q: What is the pH of a 0.117 M solution of acid HA if the ionization constant (KA) of this acid is 0.42
A:
Q: Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this…
A: Since, From the definition, Brønsted-Lowry acid are proton donar and Brønsted-Lowry base are proton…
Q: Draw the predominant product(s) of the following reactions including stereochemistry when it is…
A: When alkene or Alkyne reacts with hydrogen halide it follow morkovnikov rule and nucleophilic part…
Q: monochlorinated products relative to di-and trichlorinated products can be increased by A. adding…
A: 4) In the reaction of ethane with chlorine under photolytic condition, the amount of monochlorinated…
Q: What peaks would you look for in the IR spectrum that will help you distinguish between serotonin…
A: The structure of serotonin and melatonin is given in the image attached
Q: OH blocks top! w рая о-он Ph excess OH H₂, Pd/C 1. 03, -78 C 2. (CH3)2S ** 1) BH3 af sam 2) NaOH,…
A: Peracid is used for epoxidation. catalytical reduction of alkenes give alkane oxonolysis of alkenes…
Q: Give the expected products from each of the following reactions. a) b) NaOH H₂O 요 -CHO + CH₂CH₂C-H…
A:
Q: draw a lewis diagram for C2H5OC2H5
A: Since, Lewis structure is the representation of the valence electrons in the molecule. Thus,
Q: 22. Write short notes on any three of the following : তলত দিয়াবোৰৰ যি কোনো তিনিটাৰ চমু টোকা লিখা :…
A:
Q: 43. Identify the reagent that will produce the following brominated product in greatest amount. Br…
A: since you posted a question with multiple subparts, we will solve the first three subparts for you.…
Q: Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds below.…
A: Rules to assign oxidation number :★ The oxidation number of free and neutral element is always…
Q: * Calculate the energy electron from of ΔΕΞ Is this absorption for the transition of an the h-8 to…
A:
Q: Determine the major product (include stereochemistry)
A: Ozonlysis
Q: Calculate the pH of the solution given the: 0.04M HCl, 100% ionized
A:
Q: Balance the following redox reaction in acidic solution using the half reactions method. CuS (s) +…
A:
Q: One of the main acidic components of acid rain is sulfuric acid, H₂SO4. Assuming sulfuric acid is…
A:
Q: Use the References to access important values if needed for this question. A 7.33 gram sample of…
A:
Q: How are metalloproteins structures determined? What methods are used? Describe each in a few…
A: Metalloproteins - the metals that are useful in making of protein via interacting their captions…
Q: 1) 2) Br. CI HO DME DMSO Explain (in no less than 2 sentences) why these reactions proceed via SN2…
A:
Q: Calculate the pH given the concentration: OH = 4.0.x 10-2
A: pH is equal to the negative logarithmic of hydrogen ion concentration
Q: Imagine an alternate universe where the value of the Planck constant is 6.62607 x 10-37J.s. In that…
A: Quantum deals with microscopic species
Q: Create the following "straight chain" alkanes and fill in their names. CH4 fill in the blank…
A: •Here some straight chain alkane are given. •For naming of alkane we add suffix "ane" in end of root…
Q: At what temperature in Kelvin will 50.01 moles of neon at a pressure of 1.01 atm occupy a volume of…
A:
Q: Why do enzymes contain metals? What general reaction types to metalloenzymes catalyze? What is meant…
A:
Q: Dilution A chemist makes 220. mL of aluminum chloride (AICI3) working solution by adding distilled…
A: The concentration of a solution decreases upon dilution. This is because concentration is inversely…
Q: For the reaction 2H₂O(1) 2H₂(g) + O₂(g) AH = 572 kJ and AS° = 327 J/K At standard conditions, this…
A:
Q: Calculate the volume of 70% Isopropyl alcohol needed to prepare 50.00 mL of 5.0%, 18.0%, and 28.0%…
A:
Q: c) Aqueous Ba(OH)₂ is reacted with two equivalents of gaseous HC1 Enter your chemical notation here
A: When an acid reacts with a base results in the formation of salt and water this type of reaction is…
Q: 7.7 Predict the product of the reaction. HCI
A: To find out the product now we have to look out the mechanism for this reaction. Actually this…
Q: 52. In a concerted or single step reaction, A. the rate of forward reaction is equal to the rate of…
A:
Q: When aqueous solutions of copper(II) sulfate and ammonium carbonate are combined, solid copper(II)…
A:
Q: Information: Each spectra below was obtained from a pure compound. Mass Spectrum parent peaks (M)…
A: The proton and carbon NMR gives diffrent peaks for hydrogens and carbon having different chemical…
Q: Consider the titration of a 60.0 mL of 0.321 M weak acid HA (Ka = 4.2 x 10⁻⁶) with 0.400 M KOH. 1.…
A: Since HA is a weak acid, hence it will dissociate into its conjugate base and hydrogen ion. Since…
Q: ALEKS-Lara Althawad-Lear X…
A:
Q: + HC1
A:
Q: Propose an efficient synthesis for the following transformation: Br The transformation above can be…
A:
Q: 1. A sample of propane (C3H3) gas was burned with excess oxygen gas. If 4.20 L of CO₂ were produced…
A:
Q: An aqueous solution of lead acetate, , contains 12.1 grams of lead acetate and 17.3 grams of water.…
A:
Q: Write a valid Lewis structure of the compound with the elemental composition C3H7I that gives the…
A: Lewis dot structure of any molecule shows the bond pair and lone pair of each atom present in that…
Q: Br of ? DBU Modify the given copy of the starting material to draw the product of this reaction. Use…
A: Given reaction is elimination reaction. DBU is strong and bulky base. HBr is eliminated from the…
Q: 4. In a van't Hoff plot (InK vs. T-¹), the slope of the line was -5.22 x10³ K. What is AH° for this…
A: Van't Hoff equation shows the relation between equilibrium constant with respect to the temperature.…
Q: If you mixed 375 ml of 5.2 M HCO2H with 375 ml of 5.2 M CH3CO2-, what would be the final pH?
A: Given that 375 ml of 5.2 M HCO2H is mixed with 375 ml of 5.2 M CH3CO2- The the final pH of the…
Q: When applied to electrons the pauli excision principal requires what
A: When applied to electrons the pauli exclusion principal requires that there is only two electrons…
Q: Question 5 of 19 Maemillan Learni What is the concentration of a 52.45 mL solution of HBr that is…
A:
Q: Consider the reaction between an alcohol and tosyl chloride, followed by a nucleophile. Write the…
A: The condensed formula of the expected main organic product is....?
Step by step
Solved in 2 steps with 2 images

- The best liquid mixtures for separation by simple distillation need to have boiling points that differ by at least ___________ oC. 10 20 30 4010g coffee 4g sodium carbonate 40m dichloromethane 160ml distilled water coffee filter What are the principles behind caffeine being extracted from coffee using the above items? 1st sodium carbonate mixed with coffee then brought to a boil for 20min then solution/grinds are filtered. Then solution is added to a separatory funnel with 15ml of dichloromethane and two layers are observed so the bottom layer with DCM contains the caffeine, then this layer is collected in a beaker and dried out using molecular seeds. Then the solvent is brought to a boil with crude caffeine remaining and recystallized using 95% ethanol and brought to a boil again. Then the contents are vaccum filtered and allowed to dry resulting in 30mg caffeine.A mixture of 5 mL of acetone (boiling point 56 ° C) and toluene (boiling point 111 ° C) was separated by fractional distillation. At the end of the distillation, 2.5 mL of the fraction 1 and 1.6 mL of fraction 2. The percent recovery of toluene is: a.39% b.50% C.32% d.82%
- Which of the following statements is false in a simple and steam distillation? Simple distillation can help in separating homogenous mixtures of 1-propanol (BP of 97 °C) and propanone (BP of 56 °C) A liquid with high boiling point that decomposes on simple distillation may not be steam distilled for its purification. Steam distillation can help in separating immiscible mixtures with wide difference in boiling point. Simple distillation uses a homogeneous mixture while steam distillation separates heterogeneous mixture.Emmett, a sophomore chemistry major, was trying to separate an unknown mixture. His instructor told him that there was a carboxylic acid, phenol, and amine present in the mixture. He dissolved 3 g of the unknown in 10 ml of t-butyl methyl ether. He then added 10 ml of 3M sodium hydroxide to the mixture. He used a separatory funnel to separate the layers and collected the aqueous layer in an Erlenmeyer flask labeled fraction 1. Then he added 10 ml of 1.5M sodium bicarbonate to the remaining ether. He separated the layers, and collected the aqueous layer in an Erlenmeyer flask labeled fraction 2. Finally he added 10 ml of 1.5 M hydrochloric acid to the ether, and separated the aqueous later in an Erlenmeyer flask labeled fraction 3. Emmett neutralized the respective fractions. He obtained precipitate in fraction 1, and fraction 3. He ran an IR of the solid obtained in fraction 1 and saw a broad absorption at 3500-3300 cm-1 and a sharp absorption at 1710 cm-1. Fraction 3 showed…A mixture containing only 1-butanol (BP = 117 °C) and methanol (BP = 65 °C) in a 1:1 mass ratio undergoes fractional distillation with a very large number of evaporation-condensation cycles. What liquid is the first drop collected in the receiving flask? toluene methanol 1-butanol water
- Simple Distillation The temperature was 76o C when the first drop of distillate was collected. Liquid was collected and the temperature gradually rose to 124 o C. Fraction 2 was taken when liquid was distilling at 124o C. Fractional Distillation The temperature was 77o C when the first drop of distillate was collected. Liquid was collected until the temperature reached 78 o C, then the temperature dropped. The temperature of the hot plate was increased and a second fraction of liquid was collected at 126 o C. a.) Based on the boiling points observed when the fractions were collected, what is the identity of the unknown compound isolated in fraction 1 and Fraction 2?A binary mixture of benzene and toluene containing 57.47 mol% benzene is continuously distilled. The distillate contains 90.40 mol% benzene, while the bottom product contains 86.96 mol% toluene. What molar flow rate of the bottom product will correspond to 183.11 mol/h of the distillate product? Type your answer in mol/h, 2 decimal places.A mixture of these four liquids are separated using a fractional distillation: toluene, boiling point = 111 oC; acetone, boiling point = 56 oC; benzene, boiling point = 80 oC;and methanol, boiling point = 65 oC. Which liquid will distill off first? Toluene Benzene Methanol Acetone
- At 1 atm, a mixture of octane and methylbenzene boils at 135.8 degrees Celsius with a composition of 60% methylbenzene. The boiling points of methylbenzene and octane are 110.6 and 125.6 degrees Celsius, respectively. Draw a boiling point-composition diagram for the octane-methylbenzene mixture system, including all significant labels.Nitroethylene, HyC = CHNO, is a sensitive compound that must be prepared with great care. Attempted purification of nitroethylene by distillation often results in low recovery of product and a white coating on the inner walls of the distillation apparatus. Explain.Compare the simple distillation of pure hexane and binary mixture of hexane and toluene to that of a fractional distillation?

