Temperature (°C) 70 65 60 55 50 45 40 35 30 Freezing Point Determination: Pure Solvent 10 20 40 " D 60 80 100 Time (seconds) 120 140 160 Liquid cooling y = -0.3333x + 63.2 R² = 0.9889 Liquid Freezing y = -0.0255x + 44.929 R² = 0.989 ◆ Liquid Cooling Freezing
Temperature (°C) 70 65 60 55 50 45 40 35 30 Freezing Point Determination: Pure Solvent 10 20 40 " D 60 80 100 Time (seconds) 120 140 160 Liquid cooling y = -0.3333x + 63.2 R² = 0.9889 Liquid Freezing y = -0.0255x + 44.929 R² = 0.989 ◆ Liquid Cooling Freezing
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 4Q
Related questions
Question
A) When 1.0105 g of an unknown was added, the freezing point of the solution was determined to be 41.8 °C. Calculate the change in the freezing point, and then calculate the molality of the solution. The Kf of lauric acid is 3.90 °C⁄?.
B) Using the determined molality, calculate the number of moles of unknown present, and then calculate the molar mass of the unknown. 9.467 grams of Lauric acid was used
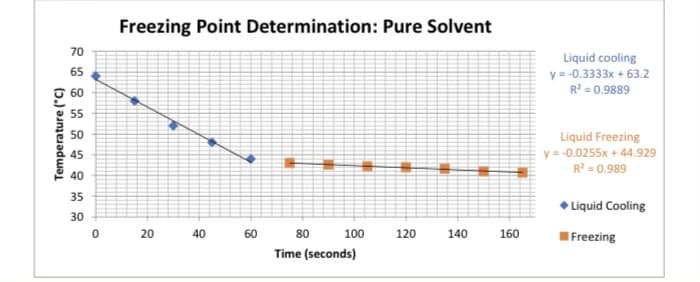
Transcribed Image Text:Temperature (°C)
70
65
60
55
50
45
40
35
30
Freezing Point Determination: Pure Solvent
10
20 40
4
D
60
100
80
Time (seconds)
120
140
160
Liquid cooling
y = -0.3333x + 63.2
R² = 0.9889
Liquid Freezing
y = -0.0255x+44.929
R² = 0.989
◆ Liquid Cooling
I Freezing
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT