Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.62PAE: 62 Ammonium dinitramide (ADN), NH4N(NO2)2, was considered as a possible replacement for aluminium...
Related questions
Question
the answer of part A is 0.721
Transcribed Image Text:Review
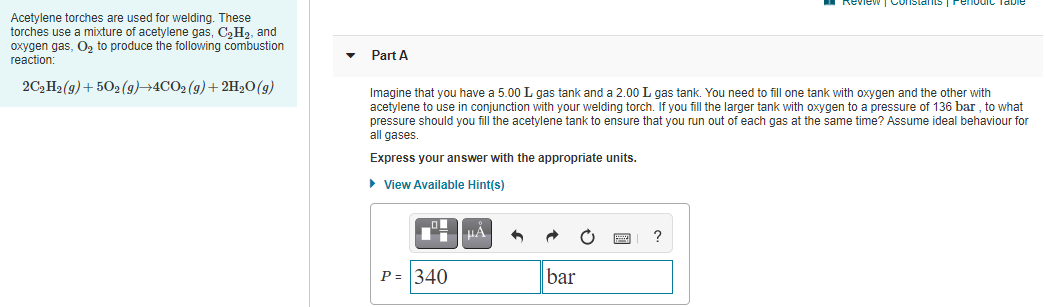
Acetylene torches are used for welding. These
torches use a mixture of acetylene gas, C,H2, and
oxygen gas, O, to produce the following combustion
reaction:
Part A
2C, H2(g)+ 502 (g)→4CO2 (g)+ 2H20(g)
Imagine that you have a 5.00 L gas tank and a 2.00 L gas tank. You need to fill one tank with oxygen and the other with
acetylene to use in conjunction with your welding torch. If you fill the larger tank with oxygen to a pressure of 136 bar , to what
pressure should you fill the acetylene tank to ensure that you run out of each gas at the same time? Assume ideal behaviour for
all gases.
Express your answer with the appropriate units.
• View Available Hint(s)
HA
P = 340
bar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning