The boiling point of ethanol CH3CH,OH is 78.50°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in ethanol is saccharin How many grams of saccharin, C-H&NOS (183.2 g/mol), must be dissolved in 220.0 grams of ethanol to raise the boiling point by 0.500 °C ? Refer to the table for the necessary boiling on freezing point constant. Solvent Formula K CC/m) Kf°C/m) Water H20 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCI3 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH;CH2OCH2CH3 2.02 g saccharin Submit Answer Retry Entire Group 9 more group attempts remaining
The boiling point of ethanol CH3CH,OH is 78.50°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in ethanol is saccharin How many grams of saccharin, C-H&NOS (183.2 g/mol), must be dissolved in 220.0 grams of ethanol to raise the boiling point by 0.500 °C ? Refer to the table for the necessary boiling on freezing point constant. Solvent Formula K CC/m) Kf°C/m) Water H20 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCI3 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH;CH2OCH2CH3 2.02 g saccharin Submit Answer Retry Entire Group 9 more group attempts remaining
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 70E: The vapor pressures of several solutions of water-propanol (CH3CH2CH2OH) were determined at various...
Related questions
Question
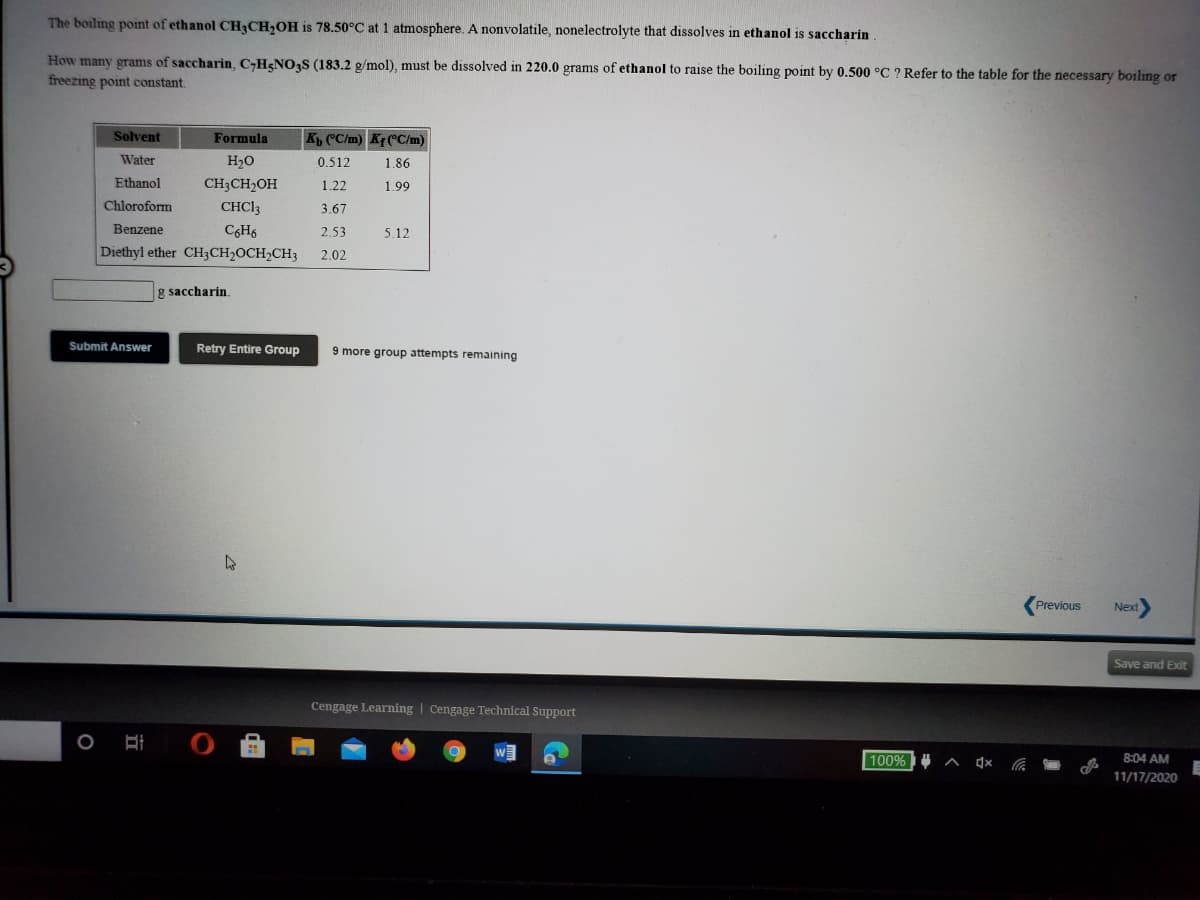
Transcribed Image Text:The boiling point of ethanol CH3CH,OH is 78.50°C at 1 atmosphere. A nonvolatile, nonelectrolyte that dissolves in ethanol is saccharin
How many grams of saccharin, C-H5NO3S (183.2 g/mol), must be dissolved in 220.0 grams of ethanol to raise the boiling point by 0.500 °C ? Refer to the table for the necessary boiling or
freezing point constant.
Solvent
Formula
K CC/m) Kf( C/m)
Water
H2O
0.512
1.86
Ethanol
CH3CH2OH
1.22
1.99
Chloroform
CHCI3
3.67
Benzene
C6H6
2.53
5.12
Diethyl ether CH3CH2OCH2CH3
2.02
g saccharin.
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Save and Exit
Cengage Learning | Cengage Technical Support
100%
8:04 AM
11/17/2020
![[Review Topics]
[References]
Use the References to access inmportant values if needed for this question.
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. The vapor pressure of diethyl ether, CH3CH2OCH,CH3, is 463.57 mm Hg at 25 °C.
In a laboratory experiment, students synthesized a new compound and found that when 26.16 grams of the compound were dissolved in 274.2 grams of diethyl ether, the vapor pressure of the
solution was 451.84 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight of this compound?
diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.
MW =
g/mol
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Save and Exit
Cengage Learning | Cengage Technical Support
8:04 AM
100% ^
11/17/2020](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd625bb90-bef1-43a1-8a4c-22731e64d06f%2Fb76230a2-993e-4685-a5cd-cbfc873726d7%2F3sfn64c_processed.jpeg&w=3840&q=75)
Transcribed Image Text:[Review Topics]
[References]
Use the References to access inmportant values if needed for this question.
The common laboratory solvent diethyl ether (ether) is often used to purify substances dissolved in it. The vapor pressure of diethyl ether, CH3CH2OCH,CH3, is 463.57 mm Hg at 25 °C.
In a laboratory experiment, students synthesized a new compound and found that when 26.16 grams of the compound were dissolved in 274.2 grams of diethyl ether, the vapor pressure of the
solution was 451.84 mm Hg. The compound was also found to be nonvolatile and a non-electrolyte.
What is the molecular weight of this compound?
diethyl ether = CH3CH2OCH2CH3 = 74.12 g/mol.
MW =
g/mol
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Save and Exit
Cengage Learning | Cengage Technical Support
8:04 AM
100% ^
11/17/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning