The freezing point of water, H₂O, is 0.000 °C at 1 atmosphere. Kf(water) = 1.86 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.14 grams of the compound were dissolved in 243.8 grams of water, the solution began to freeze at -1.369 °C. The compound was also found to be nonvolatile and a non- electrolyte. What is the molecular weight they determined for this compound? g/mol The boiling point of diethyl ether, CH3CH₂OCH₂CH3, is 34.500 °C at 1 atmosphere. K₁(diethyl ether) = 2.02 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.39 grams of the compound were dissolved in 297.6 grams of diethyl ether, the solution began to boil at 34.768 °C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound ? g/mol The boiling point of water, H₂O, is 100.000 °C at 1 atmosphere. K₂(water) = 0.512 °C/m In a laboratory experiment students synthesized a new compound and found that when
The freezing point of water, H₂O, is 0.000 °C at 1 atmosphere. Kf(water) = 1.86 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.14 grams of the compound were dissolved in 243.8 grams of water, the solution began to freeze at -1.369 °C. The compound was also found to be nonvolatile and a non- electrolyte. What is the molecular weight they determined for this compound? g/mol The boiling point of diethyl ether, CH3CH₂OCH₂CH3, is 34.500 °C at 1 atmosphere. K₁(diethyl ether) = 2.02 °C/m In a laboratory experiment, students synthesized a new compound and found that when 11.39 grams of the compound were dissolved in 297.6 grams of diethyl ether, the solution began to boil at 34.768 °C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound ? g/mol The boiling point of water, H₂O, is 100.000 °C at 1 atmosphere. K₂(water) = 0.512 °C/m In a laboratory experiment students synthesized a new compound and found that when
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
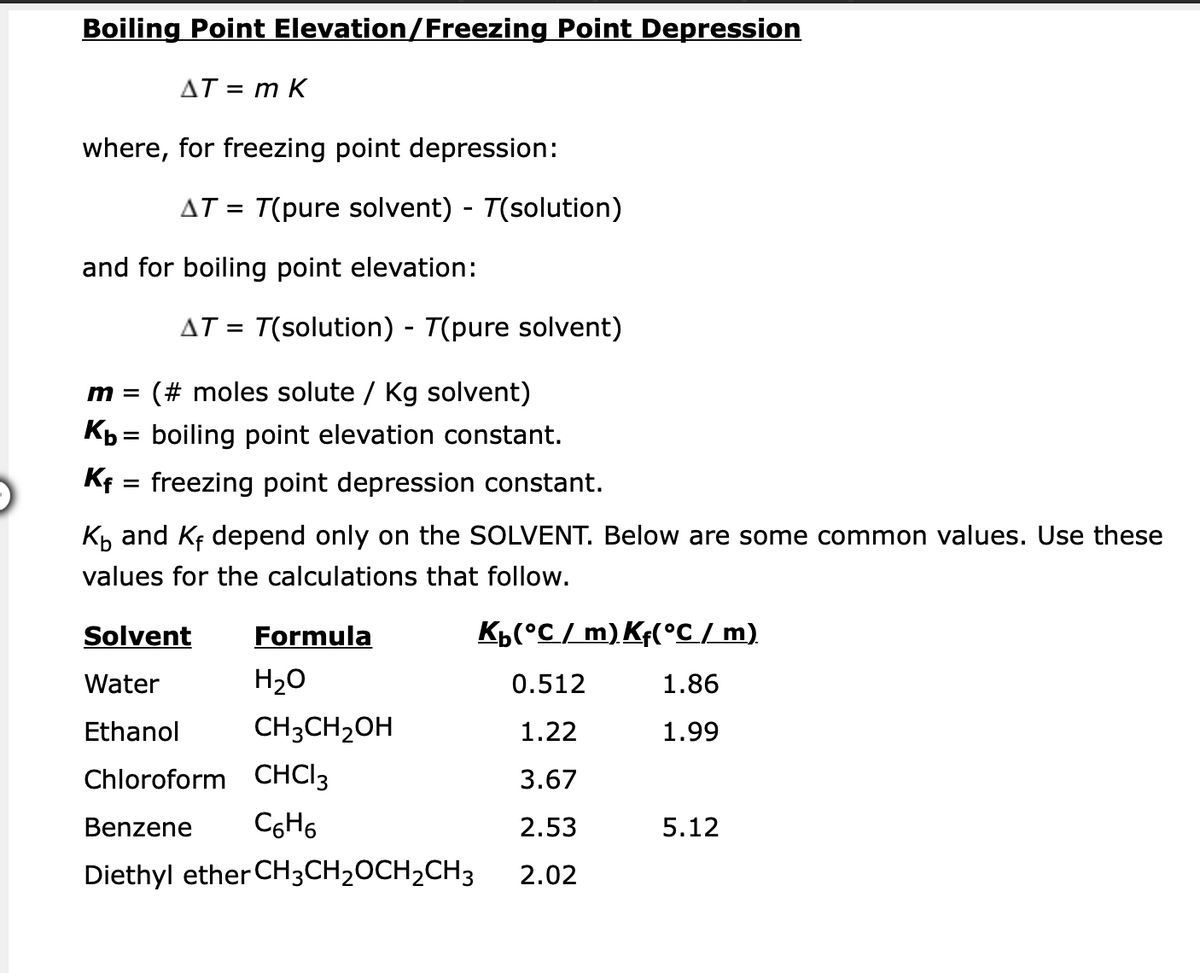
Transcribed Image Text:Boiling Point Elevation/Freezing Point Depression
AT = m K
where, for freezing point depression:
and for boiling point elevation:
m = (# moles solute / Kg solvent)
Kb = boiling point elevation constant.
Kf
=
freezing point depression constant.
K₁ and Kf depend only on the SOLVENT. Below are some common values. Use these
values for the calculations that follow.
Solvent
Formula
K₁(°C/ m) Kf(°C / m)
Water
H₂O
0.512
1.86
Ethanol
CH3CH₂OH
1.22
1.99
Chloroform CHCI 3
3.67
Benzene C6H6
2.53
5.12
Diethyl ether CH3CH₂OCH₂CH3
2.02
AT = T(pure solvent) - T(solution)
AT = T(solution) - T(pure solvent)

Transcribed Image Text:The freezing point of water, H₂O, is 0.000 °C at 1 atmosphere. Kf(water)
= 1.86 °C/m
In a laboratory experiment, students synthesized a new compound and found that when
11.14 grams of the compound were dissolved in 243.8 grams of water, the solution
began to freeze at -1.369 °C. The compound was also found to be nonvolatile and a non-
electrolyte.
What is the molecular weight they determined for this compound?
g/mol
The boiling point of diethyl ether, CH3CH₂CH₂CH3, is 34.500 °C at 1 atmosphere.
K(diethyl ether) = 2.02 °C/m
In a laboratory experiment, students synthesized a new compound and found that when
11.39 grams of the compound were dissolved in 297.6 grams of diethyl ether, the
solution began to boil at 34.768 °C. The compound was also found to be nonvolatile and
a non-electrolyte.
What is the molecular weight they determined for this compound?
g/mol
The boiling point of water, H₂O, is 100.000 °C at 1 atmosphere. K₁(water) = 0.512
°C/m
In a laboratory experiment, students synthesized a new compound and found that when
12.61 grams of the compound were dissolved in 274.3 grams of water, the solution
began to boil at 100.392 °C. The compound was also found to be nonvolatile and a non-
electrolyte.
What is the molecular weight they determined for this compound?
g/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning