The chemical formula for lithlum fluorlde Is LiF. A chemist measured the amount of lithlum fluoride produced during an experiment. She finds that 12.3 g of lithium fluorlde Is produced. Calculate the number of moles of Ilthlum fluoride produced. Round your answer to 3 significant digits. mol Submit Assic Continue 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center ip
The chemical formula for lithlum fluorlde Is LiF. A chemist measured the amount of lithlum fluoride produced during an experiment. She finds that 12.3 g of lithium fluorlde Is produced. Calculate the number of moles of Ilthlum fluoride produced. Round your answer to 3 significant digits. mol Submit Assic Continue 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center ip
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 62QAP: An air bag is deployed by utilizing the following re tion the nitrogen gas produced inflates the air...
Related questions
Question
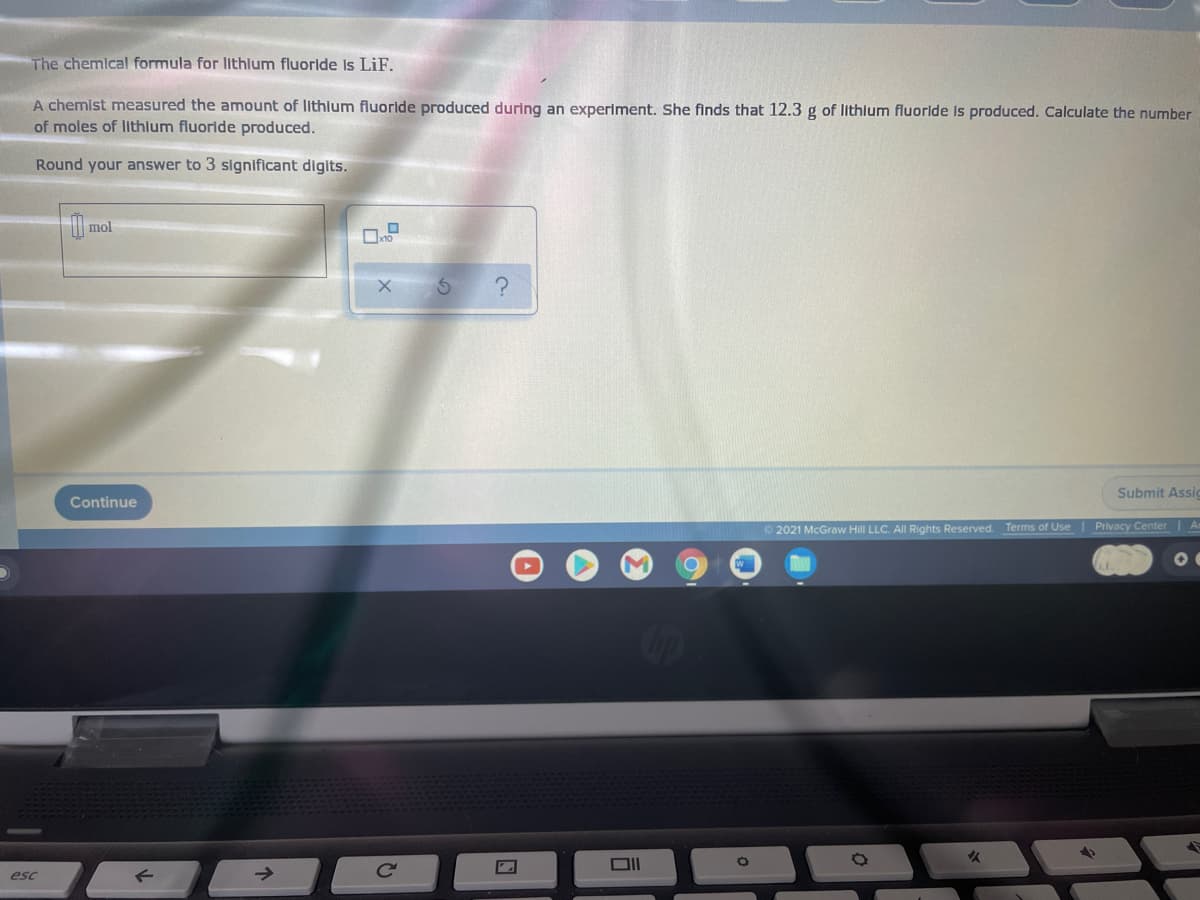
Transcribed Image Text:The chemical formula for lithlum fluorlde Is LiF.
A chemist measured the amount of lithlum fluoride produced during an experiment. She finds that 12.3 g of lithium fluoride is produced. Calculate the number
of moles of lithlum fluoride produced.
Round your answer to 3 significant digits.
| mol
Submit Assic
Continue
© 2021 McGraw Hill LLC. All Rights Reserved.
Terms of Use I Privacy Center A
esc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning