You will see similar questions like: A mineral sample is obtained from a region of the country that has high arsenic contamination. An elemental analysis yields the following elemental composition: Element Atomic Weight (g/mol) Percent Composition Cu As S 63.546 74.9216 32.065 39.3% 30.9% 29.8% What is the empirical formula of this mineral? If you need help, the simulation has step-by-step hints for you. Use the check button ( Check) to submit your answer and the hint button ( Hint) to get help with the problem. Note that each time you load this page, you will get a new version of this problem. You will practice this problem until you can get the answer correct without using the step-by- step help.
You will see similar questions like: A mineral sample is obtained from a region of the country that has high arsenic contamination. An elemental analysis yields the following elemental composition: Element Atomic Weight (g/mol) Percent Composition Cu As S 63.546 74.9216 32.065 39.3% 30.9% 29.8% What is the empirical formula of this mineral? If you need help, the simulation has step-by-step hints for you. Use the check button ( Check) to submit your answer and the hint button ( Hint) to get help with the problem. Note that each time you load this page, you will get a new version of this problem. You will practice this problem until you can get the answer correct without using the step-by- step help.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter10: Quantity Relationships In Chemical Reactions
Section: Chapter Questions
Problem 49E: A mixture of tetraphosphorus trisulfide and powdered glass is in the white tip of strike-anywhere...
Related questions
Question
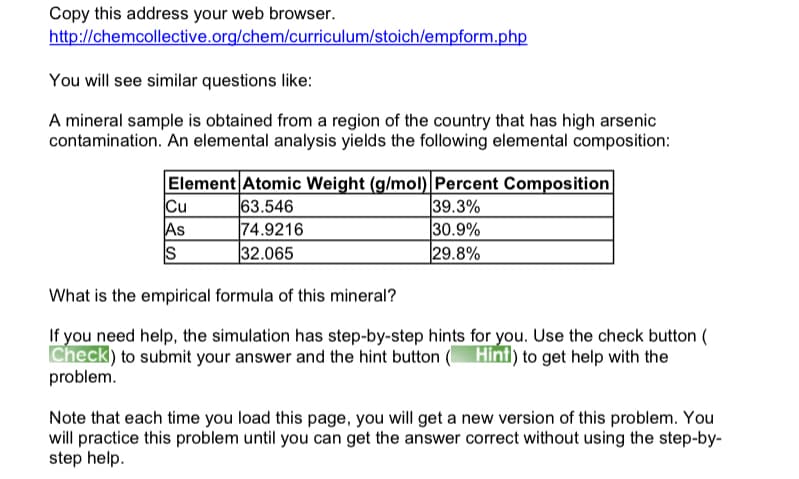
Transcribed Image Text:Copy this address your web browser.
http://chemcollective.org/chem/curriculum/stoich/empform.php
You will see similar questions like:
A mineral sample is obtained from a region of the country that has high arsenic
contamination. An elemental analysis yields the following elemental composition:
Element Atomic Weight (g/mol) Percent Composition
Cu
As
S
63.546
74.9216
32.065
39.3%
30.9%
29.8%
What is the empirical formula of this mineral?
If you need help, the simulation has step-by-step hints for you. Use the check button (
Check) to submit your answer and the hint button ( Hint) to get help with the
problem.
Note that each time you load this page, you will get a new version of this problem. You
will practice this problem until you can get the answer correct without using the step-by-
step help.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning