The density of metal #2 is 3 times greater than the density of metal #1. 52.5 g of metal #2 caused the water level in a graduated cylinder to rise by 5.3 mL. Determine the mass of metal #1 that would raise the water in a graduated cylinder by 6.7 mL. O 20 g O 21 g O 22 g O 23 g O 24 g O 25 g
The density of metal #2 is 3 times greater than the density of metal #1. 52.5 g of metal #2 caused the water level in a graduated cylinder to rise by 5.3 mL. Determine the mass of metal #1 that would raise the water in a graduated cylinder by 6.7 mL. O 20 g O 21 g O 22 g O 23 g O 24 g O 25 g
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 60RGQ: Copper: Copper has a density of 8.96 g/cm3 An ingot of copper with a mass of 57 kg (126 lb) is drawn...
Related questions
Question
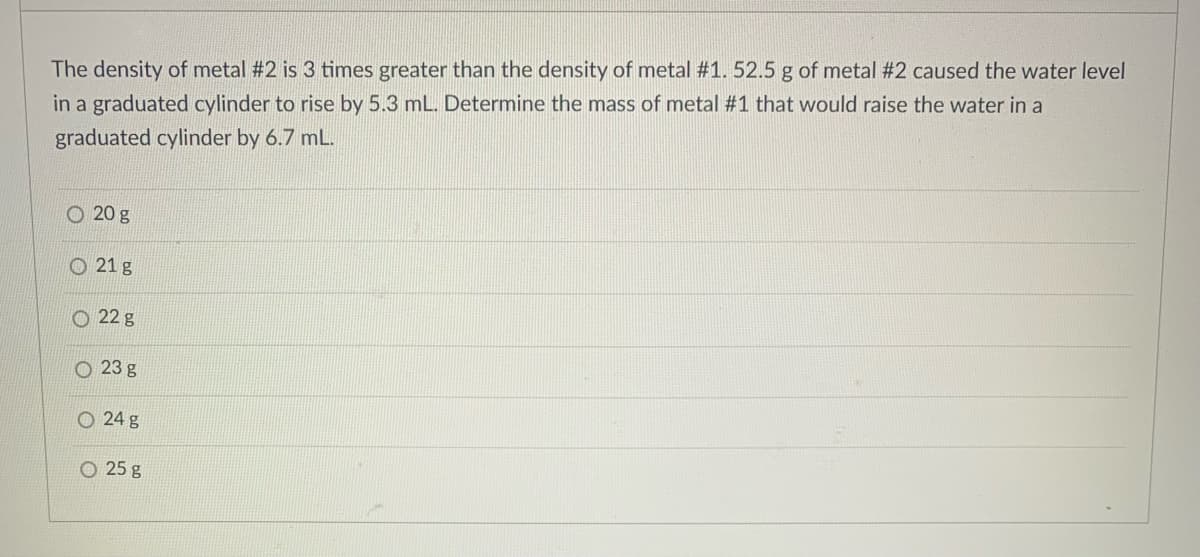
Transcribed Image Text:The density of metal #2 is 3 times greater than the density of metal #1. 52.5 g of metal #2 caused the water level
in a graduated cylinder to rise by 5.3 mL. Determine the mass of metal #1 that would raise the water in a
graduated cylinder by 6.7 mL.
O 20 g
O 21 g
O 22 g
O 23 g
O 24 g
O 25 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning