A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density. She measured the mass of the metal to be 340.3 grams. Then she dropped the metal into a measuring cup and found that it displaced 15.7 mL of water. Calculate the density of the metal. Density g/ml. Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 Nickel 8.91 Copper 8.94 Silver 10.50 Lead 11.34 Mercury 13.60 Gold 19.28 Tungsten 19.38 Platinum 21.46 This metal is most likely
A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density. She measured the mass of the metal to be 340.3 grams. Then she dropped the metal into a measuring cup and found that it displaced 15.7 mL of water. Calculate the density of the metal. Density g/ml. Densities of Some Common Substances Substance Density (g/mL) Water 1.00 Aluminum 2.72 Chromium 7.25 Nickel 8.91 Copper 8.94 Silver 10.50 Lead 11.34 Mercury 13.60 Gold 19.28 Tungsten 19.38 Platinum 21.46 This metal is most likely
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 12STP
Related questions
Question
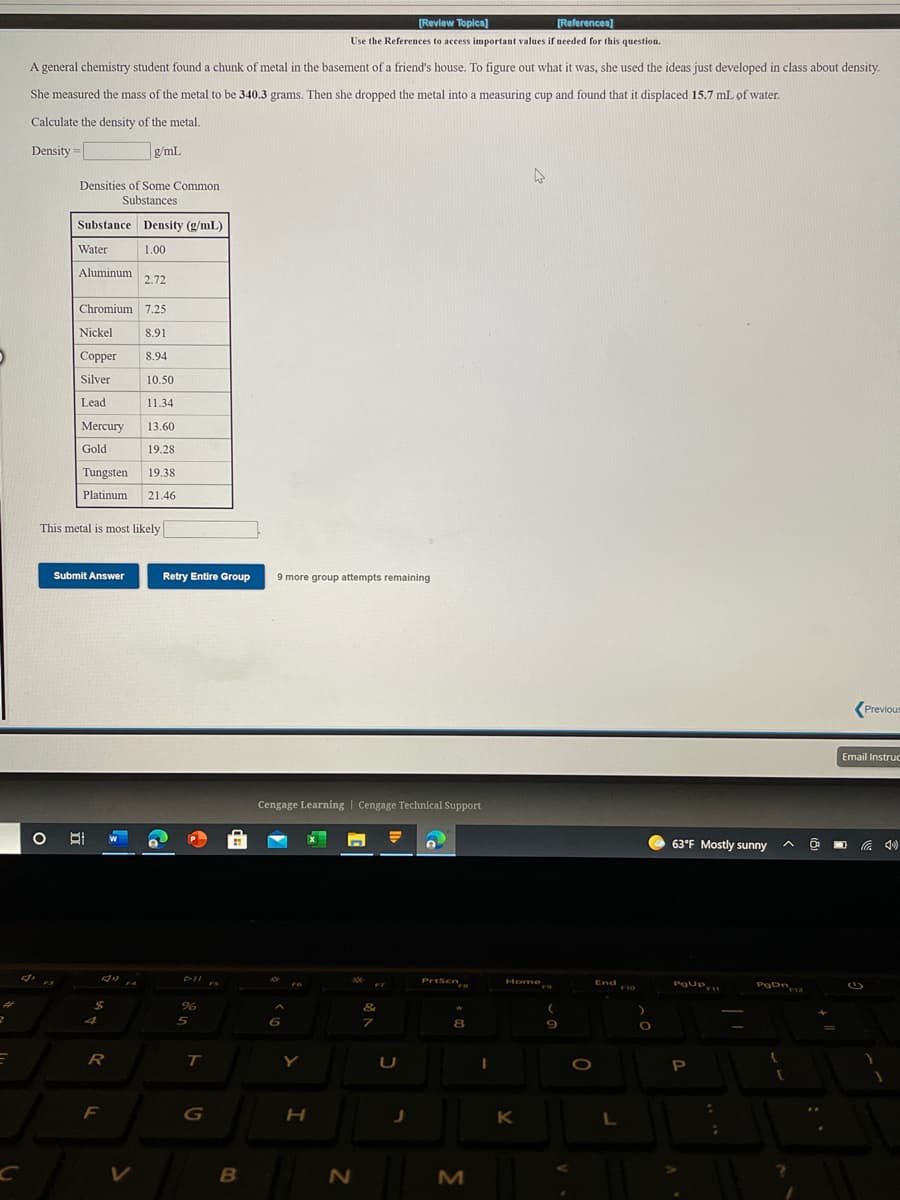
Transcribed Image Text:[Review Topics)
[References)
Use the References to access important values if needed for this question.
A general chemistry student found a chunk of metal in the basement of a friend's house. To figure out what it was, she used the ideas just developed in class about density.
She measured the mass of the metal to be 340.3 grams. Then she dropped the metal into a measuring cup and found that it displaced 15.7 mL of water.
Calculate the density of the metal.
Density =
g/mL
Densities of Some Common
Substances
Substance Density (g/mL)
Water
1.00
Aluminum
2.72
Chromium 7.25
Nickel
8.91
Copper
8.94
Silver
10.50
Lead
11.34
Mercury
13.60
Gold
19.28
Tungsten
19.38
Platinum
21.46
This metal is most likely
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Email InstruC
Cengage Learning | Cengage Technical Support
W
P.
63°F Mostly sunny
DII
PrtScn
Home
PgDn
End
F10
&
R
T.
K
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
