The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur охудen chlorine Suppose the following chemical reaction can take place in this mixture: 4 NH3(9)+5 0,(9) → 4 NO(9)+6H,O(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:
The drawing below shows a mixture of molecules: key carbon hydrogen nitrogen sulfur охудen chlorine Suppose the following chemical reaction can take place in this mixture: 4 NH3(9)+5 0,(9) → 4 NO(9)+6H,O(g) Of which reactant are there the most initial moles? Enter its chemical formula: Of which reactant are there the least initial moles? Enter its chemical formula: Which reactant is the limiting reactant? Enter its chemical formula:
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 19CR
Related questions
Question
please answer both quickly, thanks for the help
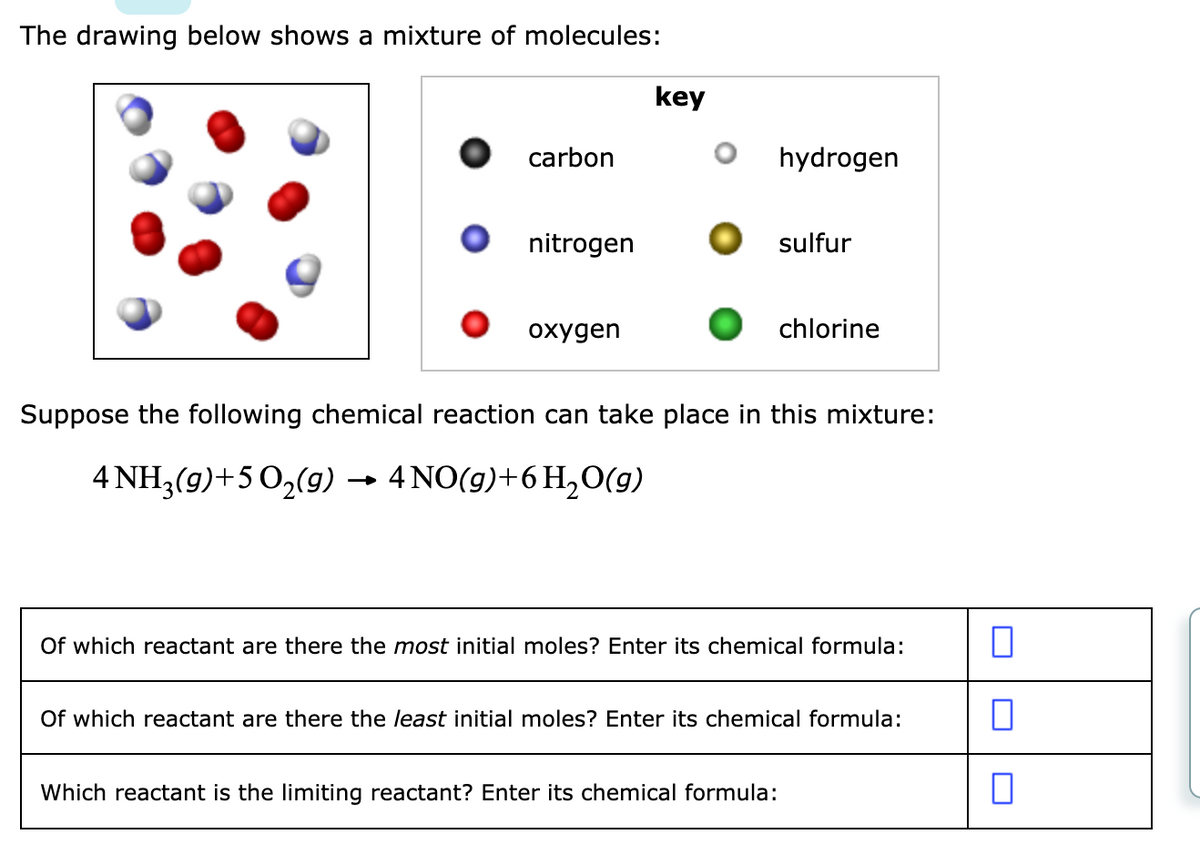
Transcribed Image Text:The drawing below shows a mixture of molecules:
key
carbon
hydrogen
nitrogen
sulfur
охудen
chlorine
Suppose the following chemical reaction can take place in this mixture:
4 NH,(9)+5 0,(9) → 4NO(9)+6H,0(9)
Of which reactant are there the most initial moles? Enter its chemical formula:
Of which reactant are there the least initial moles? Enter its chemical formula:
Which reactant is the limiting reactant? Enter its chemical formula:
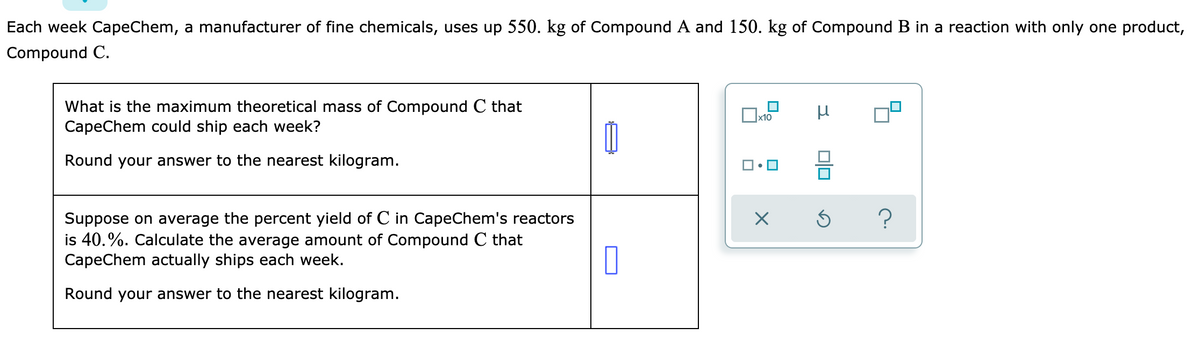
Transcribed Image Text:Each week CapeChem, a manufacturer of fine chemicals, uses up 550. kg of Compound A and 150. kg of Compound B in a reaction with only one product,
Compound C.
What is the maximum theoretical mass of Compound C that
CapeChem could ship each week?
x10
Round your answer to the nearest kilogram.
Suppose on average the percent yield of C in CapeChem's reactors
is 40.%. Calculate the average amount of Compound C that
CapeChem actually ships each week.
Round your answer to the nearest kilogram.
미□
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning