The electrolysis of CUCI(ag) produces Cu(s) and Cl2(g). What is the minimum external emf needed to drive this electrolysis under standard conditions?
The electrolysis of CUCI(ag) produces Cu(s) and Cl2(g). What is the minimum external emf needed to drive this electrolysis under standard conditions?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 160MP
Related questions
Question
please answer #5
type the answer or write it clearly so i can understand please
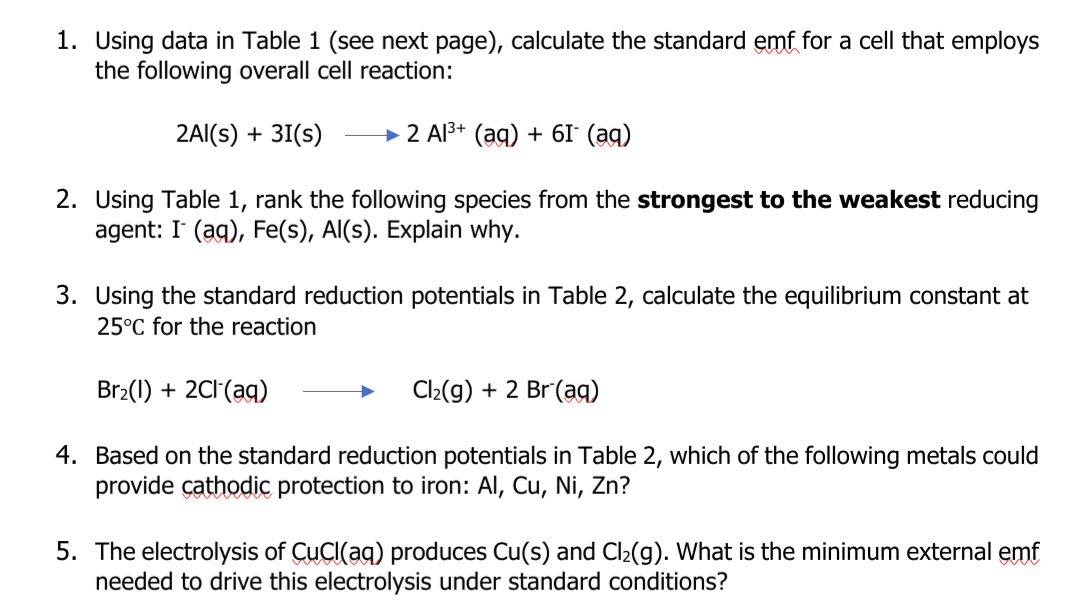
Transcribed Image Text:1. Using data in Table 1 (see next page), calculate the standard emf for a cell that employs
the following overall cell reaction:
2AI(s) + 31(s)
→ 2 Al3+ (ag) + 6I° (ag)
2. Using Table 1, rank the following species from the strongest to the weakest reducing
agent: I (ag), Fe(s), Al(s). Explain why.
3. Using the standard reduction potentials in Table 2, calculate the equilibrium constant at
25°C for the reaction
Br2(1) + 2C1(ag)
Cl2(g) + 2 Br(aq)
4. Based on the standard reduction potentials in Table 2, which of the following metals could
provide çathodic protection to iron: Al, Cu, Ni, Zn?
5. The electrolysis of CuCI(ag) produces Cu(s) and Cl2(g). What is the minimum external emf
needed to drive this electrolysis under standard conditions?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning