Identify the reaction at the anode, reaction at the cathode, the overall reaction, and the approximate potential required for the electrolysis of the following molten salts.
Identify the reaction at the anode, reaction at the cathode, the overall reaction, and the approximate potential required for the electrolysis of the following molten salts.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.59QE
Related questions
Question
Identify the reaction at the anode, reaction at the cathode, the overall reaction, and the approximate potential required for the
- CaCl2
- LiH
- AlCl3
- CrBr
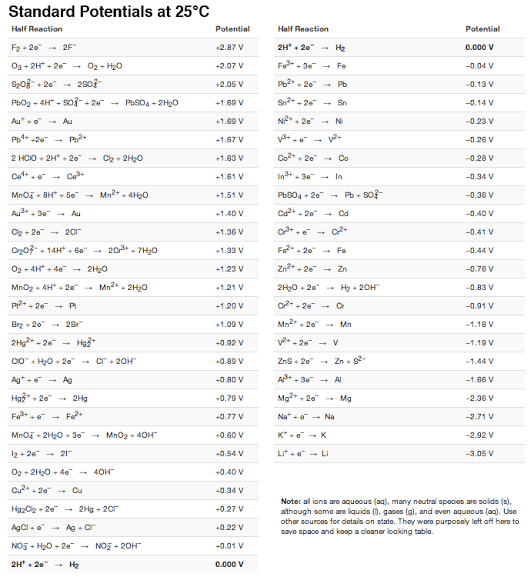
Transcribed Image Text:Standard Potentials at 25°C
Half Reaction
Potential
Half Reaction
Potential
F2 + 20 2F"
+2.87 V
2H + 20
- Hz
0.000 V
O3 + 2H* + 2e - 02 + H20
F*- 3e
+ Fe
+2.07 V
-0.04 V
Sz0+ 20 -
Pb - 20
+2.05 V
- Pb
-0.13 V
PBO2 + 4H* + So-+ 20 -
PESO4 + 2H20
+1.69 V
S2- 20
Sn
-0.14 V
Au+ Au
+1.69 V
-0.23 V
Ph+ -20 -
Po+
+1.67 V
-0.26 V
2 HCIO + 2H* + 20
Cla + 2H0
Co + 20
+1.63 V
- Co
-0.20 V
+1.61 V
- In
-0.34 V
Mno + BH+ Se
- Mn2 + 4H2O
Pb904 + 20"
Pb - so?-
-0.36 V
+1.51 V
Au+- 30"
+1.40 V
Au
+ 20"
- Cd
-0.40 V
Og - 20
- 201
+1.36 V
-0.41 V
Cr07- + 14H* + 6o" - 203 + 7H2O
Fe. 20
+1.33 V
- Fe
-0.44 V
O2 + 4H+ 4e
2H20
+1.23 V
Zn
-0.76 V
MnO2 + 4H + 20
Mn2. 2H0
+1.21 V
2H20- 20
- Hy + 20H
-0.83 V
P+- 20 - P
+1.20 V
- 20
Or
-0.91 V
Brz 20 2Br
Mn - 20
+1.00 V
Mn
-1.18 V
2Hg? + 20
+0.92 V
v*- 20 - V
-1.19 V
Co+ H0. 20
- C"+ 20H"
- Zn. s-
+0.80 V
Zns+ 20"
-1.44 V
Ag* +e + Ag
+0.80 V
A - 3e
- AL
-1.66 V
Hg3* + 20
2Hg
+0.79 V
Mg2 + 20
Mg
-2.36 V
Fe+ -
Na" + + Na
+0.77 V
-2.71 V
Mno + 2H0 . 30" - Mnoz + 4OH"
+0.60 V
K* + - K
-2.92 V
12 + 20
- 21"
+0.54 V
Li* +e - Li
-3.05 V
02 + 2120 - 4e
4OH
+0.40 V
+ Cu
+0.34 V
Note: al ions are aquoous (a), many neutral spocios are solids (2),
although some are liquids (), gases (g), and ovon aqueous (ag). Use
other sourcos for details on state. Thay wore purposely laft off hera to
savo space and koop a cloaner looking tablo.
Hg2Ce + 20 - 2Hg + 20r
+0.27 V
AgCI +o
- Ag- Cr
+0.22 V
NO5 + H20 - 20
NOE + 20H
+0.01 V
2H* + 20- Hg
0.000 V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,