Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.86E
Related questions
Question
100%
Attached are the interconnected questions which is allowed to bartleby rules. Please see image and correct the incorrect.
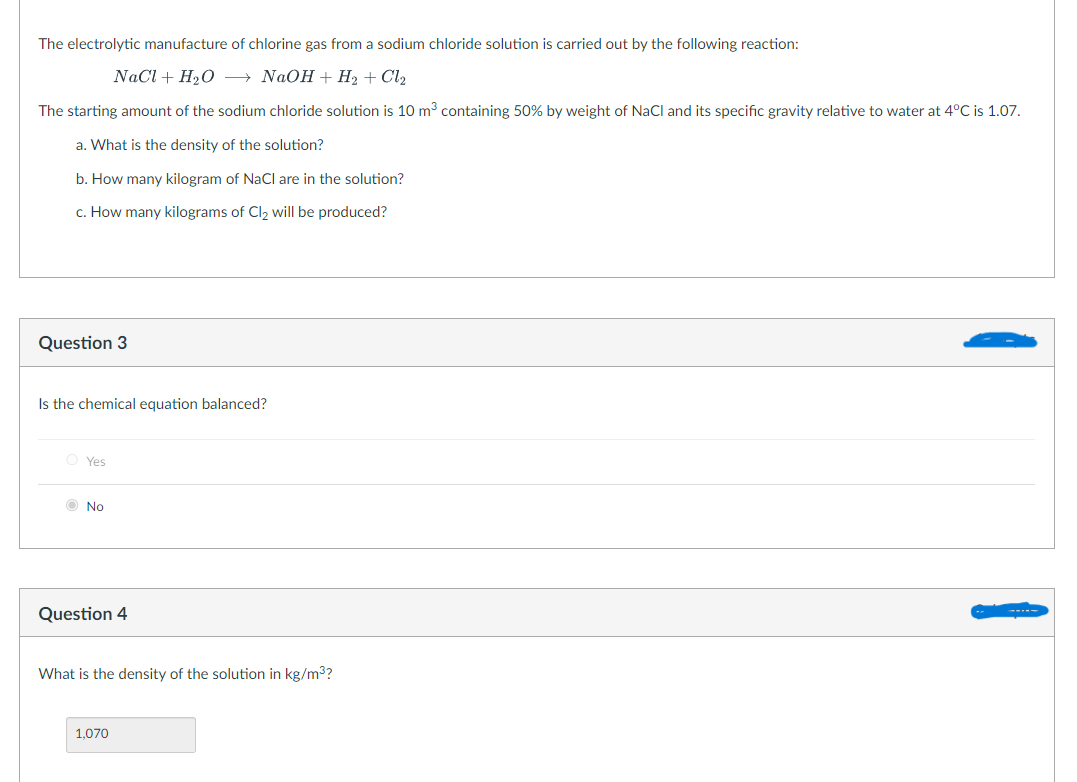
Transcribed Image Text:The electrolytic manufacture of chlorine gas from a sodium chloride solution is carried out by the following reaction:
NaCl + H₂O → NaOH + H₂ + Cl₂
The starting amount of the sodium chloride solution is 10 m³ containing 50% by weight of NaCl and its specific gravity relative to water at 4°C is 1.07.
a. What is the density of the solution?
b. How many kilogram of NaCl are in the solution?
c. How many kilograms of Cl₂ will be produced?
Question 3
Is the chemical equation balanced?
OYes
No
Question 4
What is the density of the solution in kg/m³?
1,070
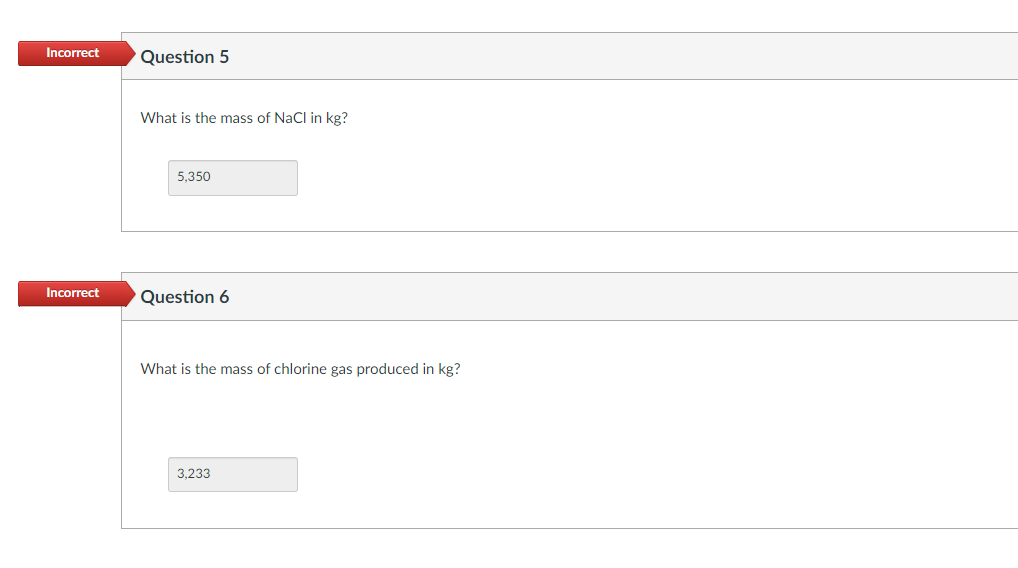
Transcribed Image Text:Incorrect Question 5
Incorrect
What is the mass of NaCl in kg?
5,350
Question 6
What is the mass of chlorine gas produced in kg?
3,233
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Hi, both answers are incorrect. Can you recheck it?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



