The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: R, E= - In this equation R, stands for the Rydberg energy, and n stands for the olo principal quantum number of the orbital that holds the electron. (You can find the value of the Rydberg energy using the Data button on the ALEKS toolbar.) Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with n=11 to an orbital with n=2. Round your answer to 3 significant digits. nm
The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: R, E= - In this equation R, stands for the Rydberg energy, and n stands for the olo principal quantum number of the orbital that holds the electron. (You can find the value of the Rydberg energy using the Data button on the ALEKS toolbar.) Calculate the wavelength of the line in the emission line spectrum of hydrogen caused by the transition of the electron from an orbital with n=11 to an orbital with n=2. Round your answer to 3 significant digits. nm
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 11PS: An energy of 3.3 1019 J/atom is required to cause a cesium atom on a metal surface to lose an...
Related questions
Question
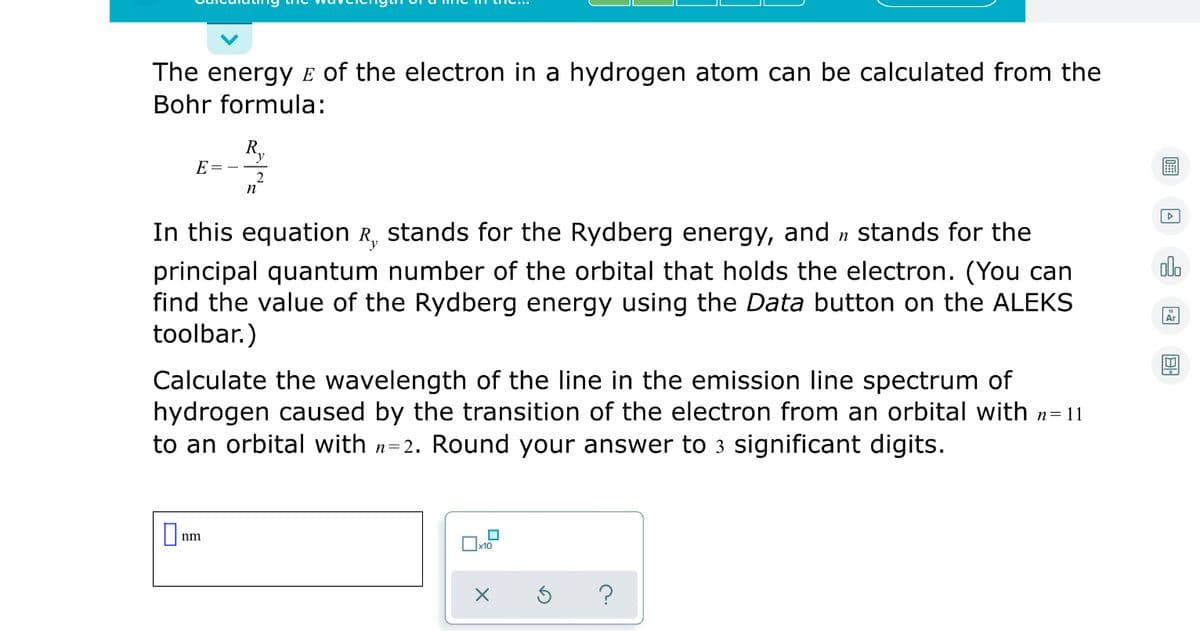
Transcribed Image Text:The energy E of the electron in a hydrogen atom can be calculated from the
Bohr formula:
R.
E=
曲
2
n
In this equation R, stands for the Rydberg energy, and n stands for the
olo
principal quantum number of the orbital that holds the electron. (You can
find the value of the Rydberg energy using the Data button on the ALEKS
toolbar.)
Ar
Calculate the wavelength of the line in the emission line spectrum of
hydrogen caused by the transition of the electron from an orbital with n=11
to an orbital with n=2. Round your answer to 3 significant digits.
O nm
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning