The equilibrium constant for a reaction can be determined by using something called a van't Hoff plot, where data for Kc and T are related by the following equation: -AH AS In Ke R R In this equation, AH is the enthalpy of reaction, AS is something called the entropy of the reaction, R = 8.314 J mol-1 K-1, and T is the temperature in Kelvin. Answer the following questions regarding this reaction. A form of a dataset where Kc was measured as the Kelvin tempetature was changed has been plotted below. Give labels for the x and y axis to indicate what has been plotted on each, and indicate the units of each axis (if there are any). y = -4300.1 x + 16.885 R° = 0.9932 According to this plot, what is Kc at 308 K? You may omit units for your Kc value and report your answer to two significant figures. Your answer_ Determine AS, to three significant figures. Include units.
The equilibrium constant for a reaction can be determined by using something called a van't Hoff plot, where data for Kc and T are related by the following equation: -AH AS In Ke R R In this equation, AH is the enthalpy of reaction, AS is something called the entropy of the reaction, R = 8.314 J mol-1 K-1, and T is the temperature in Kelvin. Answer the following questions regarding this reaction. A form of a dataset where Kc was measured as the Kelvin tempetature was changed has been plotted below. Give labels for the x and y axis to indicate what has been plotted on each, and indicate the units of each axis (if there are any). y = -4300.1 x + 16.885 R° = 0.9932 According to this plot, what is Kc at 308 K? You may omit units for your Kc value and report your answer to two significant figures. Your answer_ Determine AS, to three significant figures. Include units.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 20QAP
Related questions
Question
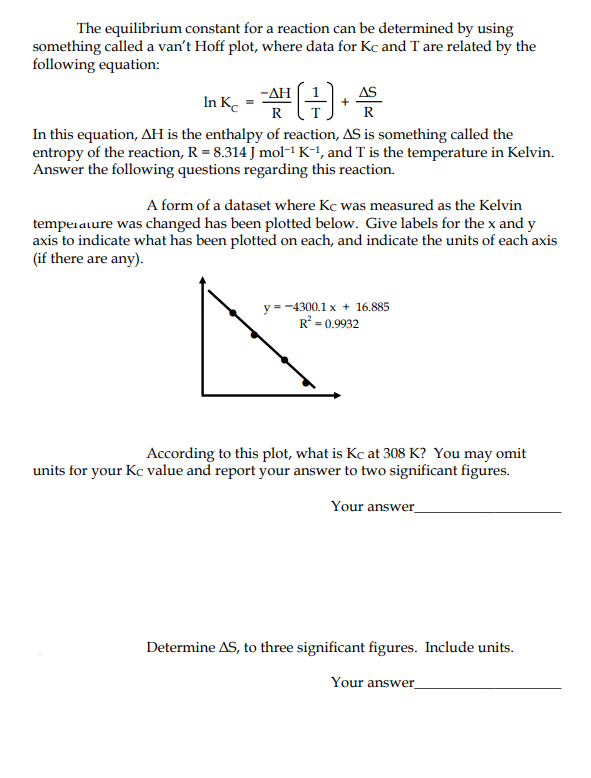
Transcribed Image Text:The equilibrium constant for a reaction can be determined by using
something called a van't Hoff plot, where data for Kc and T are related by the
following equation:
- ΔΗ
AS
In Ke
R
R
In this equation, AH is the enthalpy of reaction, AS is something called the
entropy of the reaction, R = 8.314 J mol-1 K-1, and T is the temperature in Kelvin.
Answer the following questions regarding this reaction.
A form of a dataset where Kc was measured as the Kelvin
temperature was changed has been plotted below. Give labels for the x and y
axis to indicate what has been plotted on each, and indicate the units of each axis
(if there are any).
y = -4300.1 x + 16.885
R° = 0.9932
According to this plot, what is Kc at 308 K? You may omit
units for your Kc value and report your answer to two significant figures.
Your answer_
Determine AS, to three significant figures. Include units.
Your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning