The equilibrium constant for the chemical equation N₂(g) + 3H₂(g)2NH₂(g) is Kp = 0.249 at 253 °C. Calculate the value of Ke for the reaction at 253 °C. Kc =
The equilibrium constant for the chemical equation N₂(g) + 3H₂(g)2NH₂(g) is Kp = 0.249 at 253 °C. Calculate the value of Ke for the reaction at 253 °C. Kc =
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 9.84EP: Based on the diagrams, chemical reaction, and reaction conditions depicted in Problem 9-83, which of...
Related questions
Question
How can we solve these problems?
Transcribed Image Text:General Chemistry 4th Edition
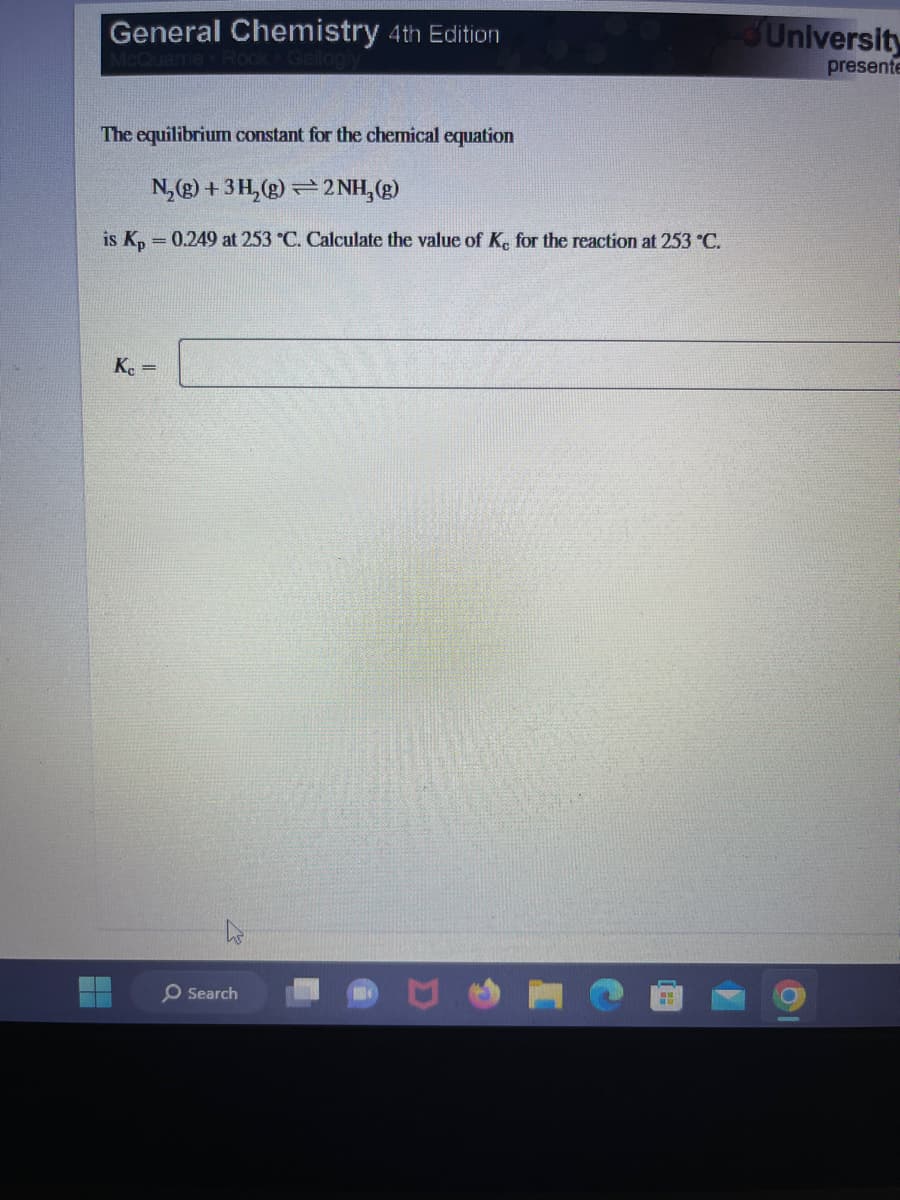
The equilibrium constant for the chemical equation
N₂(g) + 3H₂(g)2NH₂(g)
is Kp = 0.249 at 253 °C. Calculate the value of Ke for the reaction at 253 °C.
Kc =
O Search
University
presente
Transcribed Image Text:Macmillan Learning
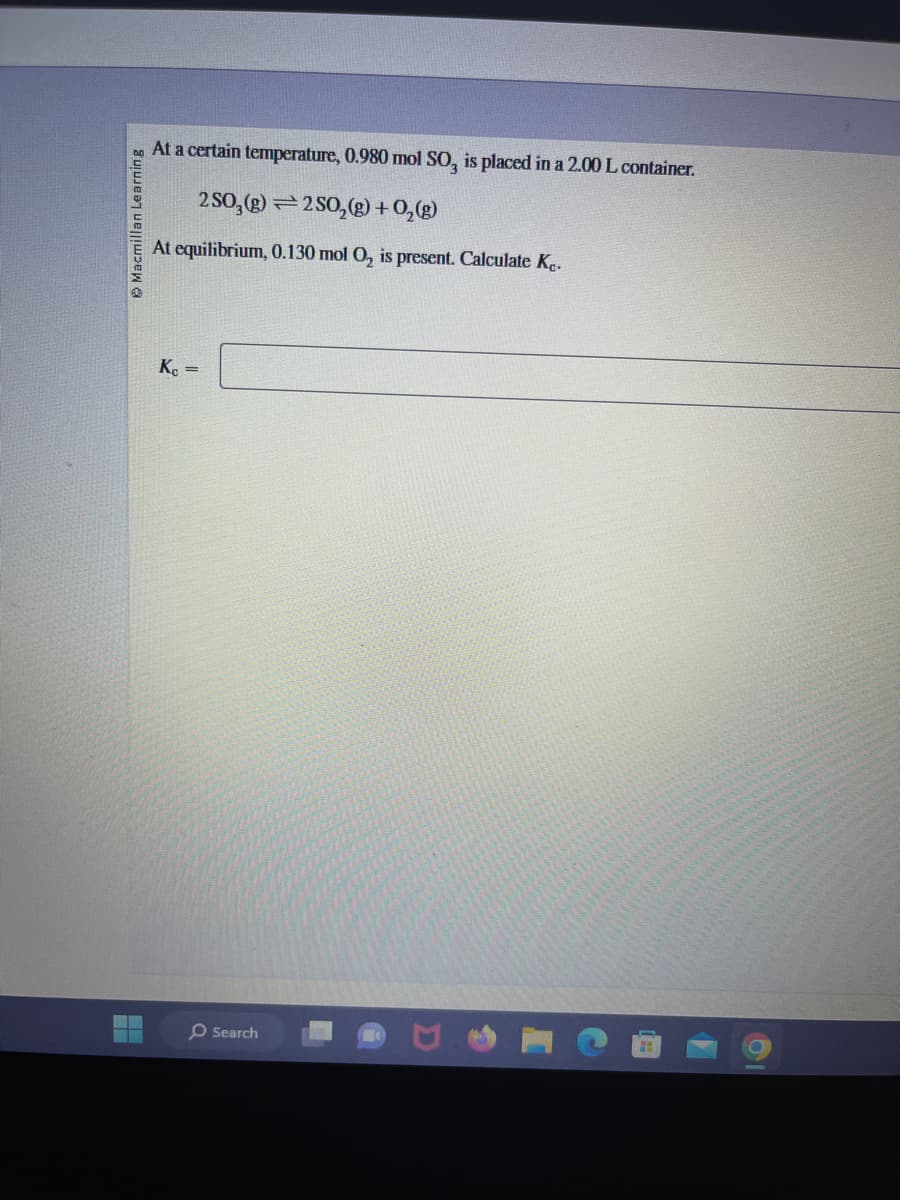
At a certain temperature, 0.980 mol SO, is placed in a 2.00 L container.
2 SO₂(g) 2 SO₂(g) + O₂(g)
At equilibrium, 0.130 mol O₂ is present. Calculate Kc.
Kc =
O Search
Expert Solution
Question -1
Given ->
N2(g) + 3H2(g) -----> 2NH3(g) Kp= 0.249
T = 253°C = 253 + 273 = 526 K

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning