The figure below shows Pb-Sn phase diagram. What is the composition of Pb and Sn at point 1? 1200 1000 Liquid 779 C (Tg). B+L 800 G 91.2 8.0 71.9 600 400 IC H. 200 20 40 60 80 100 (Pb) Composition (wt% Sn) (Sn) Callister Jr., W.D., 2007 O a. 80 wt % Sn and 20 wt % Pb O b. 10 wt % Sn and 90 wt % Pb O c. 90 wt % Sn and 10 wt % Pb O d. 20 wt % Sn and 80 wt % Pb Temperature ("C)
The figure below shows Pb-Sn phase diagram. What is the composition of Pb and Sn at point 1? 1200 1000 Liquid 779 C (Tg). B+L 800 G 91.2 8.0 71.9 600 400 IC H. 200 20 40 60 80 100 (Pb) Composition (wt% Sn) (Sn) Callister Jr., W.D., 2007 O a. 80 wt % Sn and 20 wt % Pb O b. 10 wt % Sn and 90 wt % Pb O c. 90 wt % Sn and 10 wt % Pb O d. 20 wt % Sn and 80 wt % Pb Temperature ("C)
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter11: Heat Transfer By Radiation
Section: Chapter Questions
Problem 11.8P
Related questions
Question
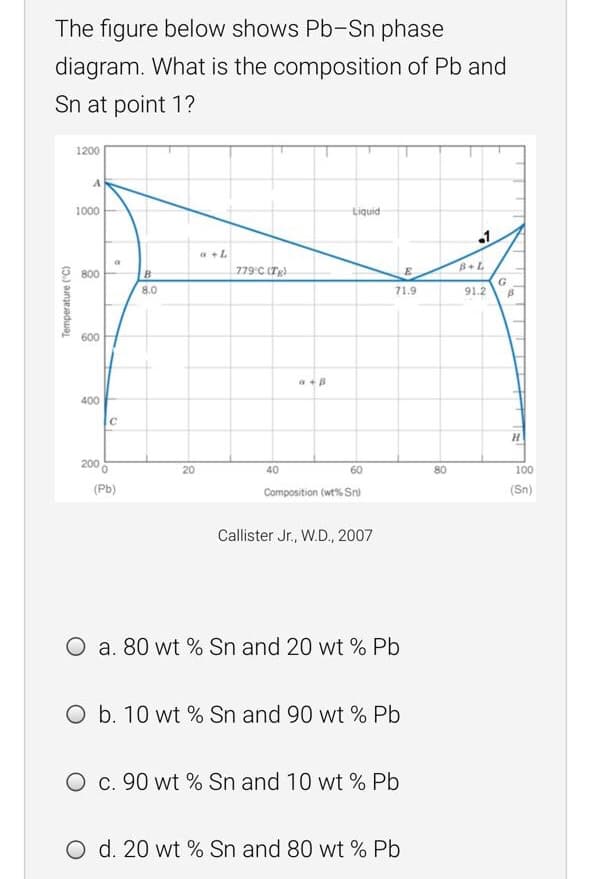
Transcribed Image Text:The figure below shows Pb-Sn phase
diagram. What is the composition of Pb and
Sn at point 1?
1200
1000
Liquid
a +L
779 C (Tg)
B+L
6 800
8.0
71.9
G
91.2
600
400
200
20
40
60
80
100
(Pb)
Composition (wt% Sni
(Sn)
Callister Jr., W.D., 2007
O a. 80 wt % Sn and 20 wt % Pb
O b. 10 wt % Sn and 90 wt % Pb
O c. 90 wt % Sn and 10 wt % Pb
O d. 20 wt % Sn and 80 wt % Pb
Temperature ("C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning