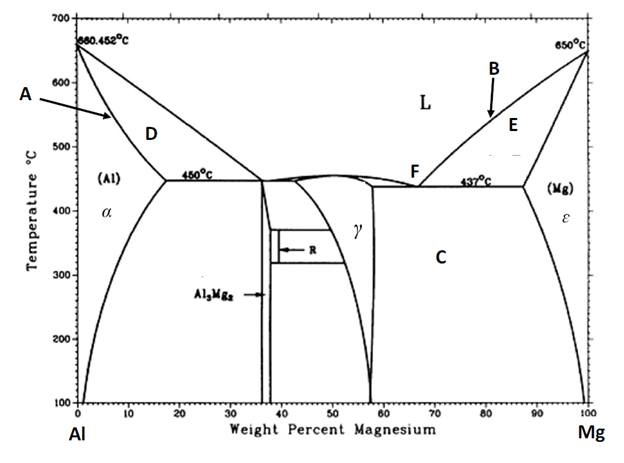
Referring to Figure 1, please answer the following [NOTE: SHOW YOUR WORK on Figure 1]: a. provide the specific name for the phase boundary lines denoted by A and B. b. identify the phases present at equilibrium in the phase fields denoted by C, D, and E. c. identify the specific name for the point denoted by F.
Referring to Figure 1, please answer the following [NOTE: SHOW YOUR WORK on Figure 1]:
a. provide the specific name for the phase boundary lines denoted by A and B.
b. identify the phases present at equilibrium in the phase fields denoted by C, D, and E.
c. identify the specific name for the point denoted by F.
d. What is the maximum solid solubility of Mg in Al? At what temperature does it occur?
e. An Al-Mg alloy (10 wt% Mg, 90 wt% Al) is heated slowly (to insure equilibrium) from a temperature of 200 C:
i. At what temperature does the first liquid phase form?
ii. What is the composition of the liquid phase at the temperature in part i.?
iii. At what temperature does complete melting (no solid phase remaining) occur?
iv. What is the composition of the last solid remaining prior to complete melting?
f. Using the equilibrium phase diagram of Figure 1, identify the phases present, their compositions, and their relative mass fractions at equilibrium for a 70 wt% Mg – 30 wt% Al alloy held at 300 C.
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

What is the maximum solid solubility of Mg in Al? At what temperature does it occur?
e. An Al-Mg alloy (10 wt% Mg, 90 wt% Al) is heated slowly (to insure equilibrium) from a temperature of 200 C:
i. At what temperature does the first liquid phase form?
ii. What is the composition of the liquid phase at the temperature in part i.?
iii. At what temperature does complete melting (no solid phase remaining) occur?
iv. What is the composition of the last solid remaining prior to complete melting?
f. Using the equilibrium phase diagram of Figure 1, identify the phases present, their compositions, and their relative mass fractions at equilibrium for a 70 wt% Mg – 30 wt% Al alloy held at 300 C.








