The flue gas analysis by volume for burning of C,H14 is as follows: co, = 8.5%, CO = 7.8%, and N, = 83.7% Calculate (a) the air-fuel ratio required for chemically correct combustion, and (b) the air-fuel (A:F) ratio as a percentage of the chemically correct mixture A:F ratio.
The flue gas analysis by volume for burning of C,H14 is as follows: co, = 8.5%, CO = 7.8%, and N, = 83.7% Calculate (a) the air-fuel ratio required for chemically correct combustion, and (b) the air-fuel (A:F) ratio as a percentage of the chemically correct mixture A:F ratio.
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter32: Oil Heat
Section: Chapter Questions
Problem 12RQ: When incomplete combustion occurs, what additional by-products of combustion are created?
Related questions
Question
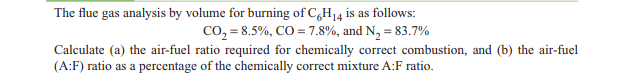
Transcribed Image Text:The flue gas analysis by volume for burning of C,H14 is as follows:
co, = 8.5%, CO = 7.8%, and N, = 83.7%
Calculate (a) the air-fuel ratio required for chemically correct combustion, and (b) the air-fuel
(A:F) ratio as a percentage of the chemically correct mixture A:F ratio.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning