The following is the strategy in modern synthesis of indigo dye in industry. A, formaldehyde and hydrogen cyanide converted into N-phenylglycinonitrile at 85 ° Hydrolysis takes place in aqueous alkali at 100 °C. The sodamide for the next stage is obtained by passing ammonia over molten sodium. The dried salt of phenylglycine (B) is dissolved in a eutectic, and therefore stirrable, mixture of sodium and potassium hydroxides at 220 °C and portions of molten sodamide are added. The reaction leads to sodium indoxylate, which undergoes oxidative hydrolysis to give indigo (yield of 84 %). CN NaOH/KOH A+CH₂O + HCN B + NH3 85 °C H₂O 100 °C 2 NaNH. NaOH/KOH 220 °C ONa NNa H₂0.0₂ 80-90 °C
The following is the strategy in modern synthesis of indigo dye in industry. A, formaldehyde and hydrogen cyanide converted into N-phenylglycinonitrile at 85 ° Hydrolysis takes place in aqueous alkali at 100 °C. The sodamide for the next stage is obtained by passing ammonia over molten sodium. The dried salt of phenylglycine (B) is dissolved in a eutectic, and therefore stirrable, mixture of sodium and potassium hydroxides at 220 °C and portions of molten sodamide are added. The reaction leads to sodium indoxylate, which undergoes oxidative hydrolysis to give indigo (yield of 84 %). CN NaOH/KOH A+CH₂O + HCN B + NH3 85 °C H₂O 100 °C 2 NaNH. NaOH/KOH 220 °C ONa NNa H₂0.0₂ 80-90 °C
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section: Chapter Questions
Problem 19.65P
Related questions
Question
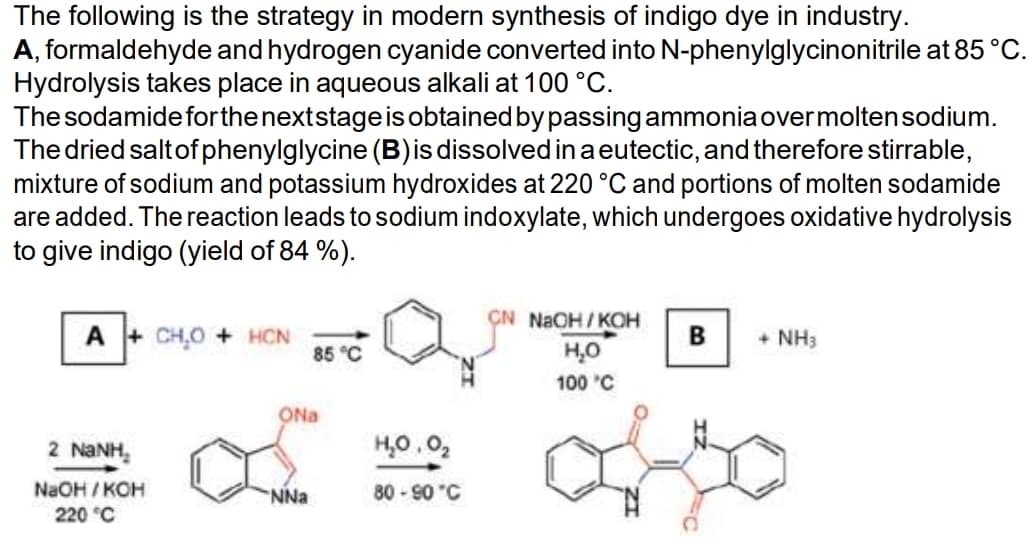
Transcribed Image Text:The following is the strategy in modern synthesis of indigo dye in industry.
A, formaldehyde and hydrogen cyanide converted into N-phenylglycinonitrile at 85 °C.
Hydrolysis takes place in aqueous alkali at 100 °C.
The sodamide for the next stage is obtained by passing ammonia over molten sodium.
The dried salt of phenylglycine (B) is dissolved in a eutectic, and therefore stirrable,
mixture of sodium and potassium hydroxides at 220 °C and portions of molten sodamide
are added. The reaction leads to sodium indoxylate, which undergoes oxidative hydrolysis
to give indigo (yield of 84 %).
CN NaOH/KOH
A+CH₂O + HCN
B + NH3
85 °C
H₂O
100 °C
2 NaNH.
NaOH/KOH
220 °C
ONa
NNa
H₂0.0₂
80-90 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning