The following Lewis representation depicts a reaction between a group metal and a group nonmetal. x2+ + 2 In this representation, each O atom loses electron(s) and each O atom gains electron(s). The bonds that form in the product are
The following Lewis representation depicts a reaction between a group metal and a group nonmetal. x2+ + 2 In this representation, each O atom loses electron(s) and each O atom gains electron(s). The bonds that form in the product are
Chapter2: Resonance Structures
Section: Chapter Questions
Problem 18EQ: The cyclohexane carboxylate anion has a Lewis structure Pushing a pair of unshared electrons away...
Related questions
Question
The first two drop downs are either:
metal or nonmetal
The last drop down is:
ionic or covalent or metallic
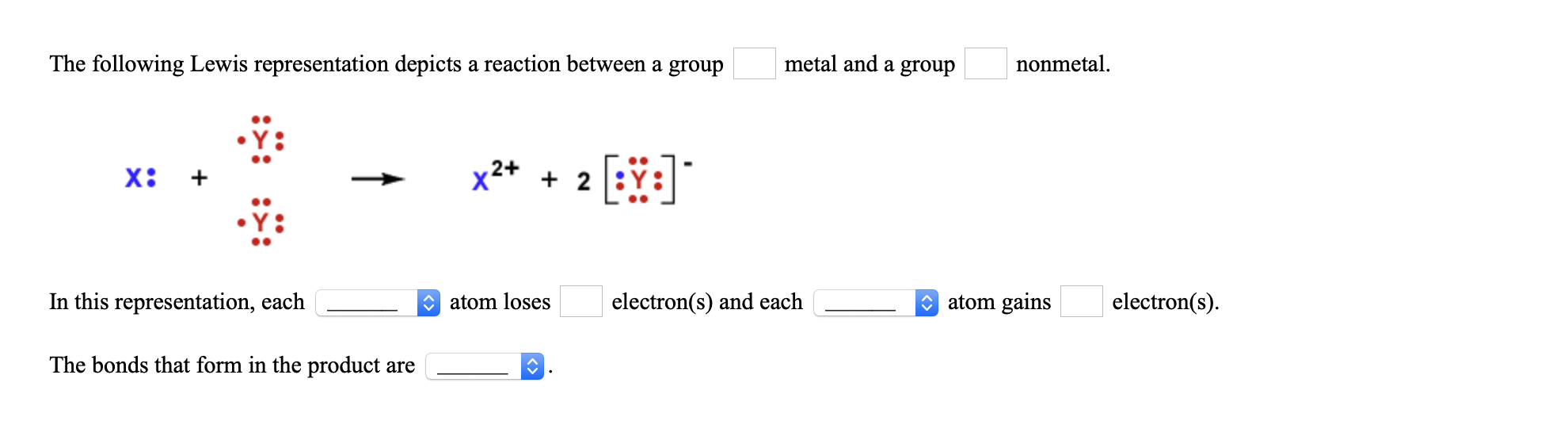
Transcribed Image Text:The following Lewis representation depicts a reaction between a group
metal and a group
nonmetal.
x2+ + 2
In this representation, each
O atom loses
electron(s) and each
O atom gains
electron(s).
The bonds that form in the product are
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning



General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning