
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 18EQ
The cyclohexane carboxylate anion has a Lewis structure
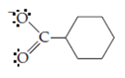
Pushing a pair of unshared electrons away from the negatively charged oxygen atom and, at the same time, pushing a pair of pi electrons toward the other oxygen will generate a second resonance structure. Thus,

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Demonstrate the concept of resonance and formal charge by drawing ALL resonance forms of the phosphate anion and labeling the formal charge on each atom on one of the structures. Determine the bond order in phosphate.
Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol.
(d) Using average bond enthalpies, estimate H for the formation of gaseous phosgene from CO(g) and Cl2(g).
In developing a Lewis structure for NO +, the nitroxonium ion, how many valence electrons must you account for?
Chapter 2 Solutions
Pushing Electrons
Ch. 2 - One Lewis structure for the 2-butenyl cation is...Ch. 2 - Prob. 2EQCh. 2 - One structure for the conjugate acid of acetone...Ch. 2 - Similarly, a resonance structure for the conjugate...Ch. 2 - Prob. 5EQCh. 2 - Pairs of unshared electrons can be pushed. One...Ch. 2 - One structure for the acetoxonium ion is Clearly,...Ch. 2 - Prob. 8EQCh. 2 - There are no important resonance structures for...Ch. 2 - Prob. 10EQ
Ch. 2 - Prob. 11EQCh. 2 - Prob. 12EQCh. 2 - Prob. 13EQCh. 2 - Prob. 14EQCh. 2 - Prob. 15EQCh. 2 - Prob. 16EQCh. 2 - Prob. 17EQCh. 2 - The cyclohexane carboxylate anion has a Lewis...Ch. 2 - One Lewis structure for the enolate anion of...Ch. 2 - Prob. 20EQCh. 2 - Prob. 21EQCh. 2 - Prob. 22EQCh. 2 - Prob. 23EQCh. 2 - Prob. 24EQCh. 2 - Prob. 25EQCh. 2 - Prob. 26EQCh. 2 - Prob. 27EQCh. 2 - Prob. 28EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One Lewis structure for the enolate anion of acetaldehyde is Pushing the pair of unshared electrons on the carbon atom away from the center of negative charge and pushing the pi electrons of the carbon-oxygen double bond to the oxygen atom generates a second resonance structure. Thus,arrow_forwardSeveral Lewis structures can be written for perbromate ion, , the central Br with all single Br—O bonds, or with one, two, or three Br=O double bonds. Draw the Lewis structures of these possible resonance structures, and use formal charges to predict which makes the greatest contribution to the resonance hybrid.arrow_forwardWhy do polyatomic ions have different bond orders than the acids they are associated with, and what causes this change?arrow_forward
- Please give the following for NO-2 Lewis Structure Hybridization of the Central atom Electron pair geometry Molecular geometry, And whether it can create resonance structures. Thank youarrow_forwardA 0.325 g sample of a gaseous hydrocarbon (compound containing carbon and hydrogen only) occupies a volume of 193 mL at 749 mmHg and 26.1°C. Determine the molecular mass, and write a plausible Lewis structure for this hydrocarbon.arrow_forwardSelect structure in which oxygen DOES have a formal charge of +1?arrow_forward
- In detail, define the terms endothermic and exothermic in relation to chemical reactions and giving relevant examples. Explain in detail the difference between the two processes in terms of bond making and bond breaking.arrow_forwardcalculate the formal charge on the oxygen in the structurearrow_forwardWhich of the Lewis structures for NO is dominant based on analysis of the formalcharges?arrow_forward
- Valine is an amino acid with this Lewis structure: Write the Lewis structure for the zwitterion form of valine.arrow_forwardDerive Lewis structures for the compounds below. Furanarrow_forwardTribromide, Br3, and triiodide, I3, ions are often found in aqueous solutions, but trifluoride ion, F3, is so rare that its bond strength was only measured in 2000. Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY