The following sets of measurement for the density of a small cylinder of aluminum were given. The 'accepted' density of aluminium is 2.702 g/cm3. SET A SET B 2. 240 g/cm³ 2.700 g/cm³ 2. 690 g/cm3 2.705 g/cm3 2. 450 g/cm³ 2.703 g/cm³ 2. 150 g/cm3 2.701 g/cm3 1. Calculate the average value for each set of density. 2. Calculate the % error for each set of values. 3. Compare the average value for each set with the accepted value: Which student's data is more accurate? Which student's data is more precise? 4. Compare the percentage error for each set: Which student's data is more accurate? Which student's data is more precise?
The following sets of measurement for the density of a small cylinder of aluminum were given. The 'accepted' density of aluminium is 2.702 g/cm3. SET A SET B 2. 240 g/cm³ 2.700 g/cm³ 2. 690 g/cm3 2.705 g/cm3 2. 450 g/cm³ 2.703 g/cm³ 2. 150 g/cm3 2.701 g/cm3 1. Calculate the average value for each set of density. 2. Calculate the % error for each set of values. 3. Compare the average value for each set with the accepted value: Which student's data is more accurate? Which student's data is more precise? 4. Compare the percentage error for each set: Which student's data is more accurate? Which student's data is more precise?
MATLAB: An Introduction with Applications
6th Edition
ISBN:9781119256830
Author:Amos Gilat
Publisher:Amos Gilat
Chapter1: Starting With Matlab
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
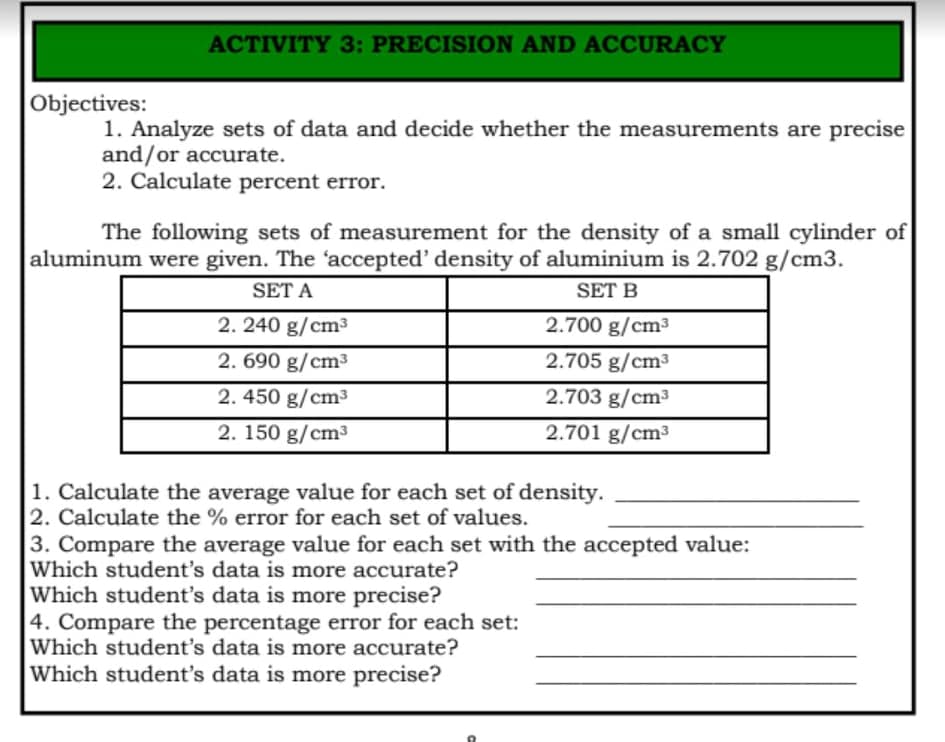
Transcribed Image Text:ACTIVITY 3: PRECISION AND ACCURACY
Objectives:
1. Analyze sets of data and decide whether the measurements are precise
and/or accurate.
2. Calculate percent error.
The following sets of measurement for the density of a small cylinder of
aluminum were given. The 'accepted' density of aluminium is 2.702 g/cm3.
SET A
SET B
2. 240 g/cm³
2.700 g/cm³
2. 690 g/cm³
2.705 g/cm3
2. 450 g/cm³
2.703 g/cm³
2. 150 g/cm³
2.701 g/cm³
1. Calculate the average value for each set of density.
2. Calculate the % error for each set of values.
3. Compare the average value for each set with the accepted value:
Which student's data is more accurate?
Which student's data is more precise?
4. Compare the percentage error for each set:
Which student's data is more accurate?
Which student's data is more precise?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

MATLAB: An Introduction with Applications
Statistics
ISBN:
9781119256830
Author:
Amos Gilat
Publisher:
John Wiley & Sons Inc

Probability and Statistics for Engineering and th…
Statistics
ISBN:
9781305251809
Author:
Jay L. Devore
Publisher:
Cengage Learning

Statistics for The Behavioral Sciences (MindTap C…
Statistics
ISBN:
9781305504912
Author:
Frederick J Gravetter, Larry B. Wallnau
Publisher:
Cengage Learning

MATLAB: An Introduction with Applications
Statistics
ISBN:
9781119256830
Author:
Amos Gilat
Publisher:
John Wiley & Sons Inc

Probability and Statistics for Engineering and th…
Statistics
ISBN:
9781305251809
Author:
Jay L. Devore
Publisher:
Cengage Learning

Statistics for The Behavioral Sciences (MindTap C…
Statistics
ISBN:
9781305504912
Author:
Frederick J Gravetter, Larry B. Wallnau
Publisher:
Cengage Learning

Elementary Statistics: Picturing the World (7th E…
Statistics
ISBN:
9780134683416
Author:
Ron Larson, Betsy Farber
Publisher:
PEARSON

The Basic Practice of Statistics
Statistics
ISBN:
9781319042578
Author:
David S. Moore, William I. Notz, Michael A. Fligner
Publisher:
W. H. Freeman

Introduction to the Practice of Statistics
Statistics
ISBN:
9781319013387
Author:
David S. Moore, George P. McCabe, Bruce A. Craig
Publisher:
W. H. Freeman