The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively. 100 - MS-IU-9451 Spectrum 1 80 - 40 20 - 25 50 75 100 125 150 175 m/z 100 - Spectrum 2 MS-NU-8350 80 - 60 - 40 - 20 - 20 40 60 80 100 120 140 160 180 200 m/z 1. Assign each spectrum to one compound 2. Justify your assignment by assigning relevant signals in each spectrum. 3. Explain how you could determine which spectrum belongs to which compound. Relative Intensity Relative Intensity
The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene, respectively. 100 - MS-IU-9451 Spectrum 1 80 - 40 20 - 25 50 75 100 125 150 175 m/z 100 - Spectrum 2 MS-NU-8350 80 - 60 - 40 - 20 - 20 40 60 80 100 120 140 160 180 200 m/z 1. Assign each spectrum to one compound 2. Justify your assignment by assigning relevant signals in each spectrum. 3. Explain how you could determine which spectrum belongs to which compound. Relative Intensity Relative Intensity
Chapter12: Structure Determination: Mass Spectrometry And Infrared Spectroscopy
Section12.SE: Something Extra
Problem 49AP: The infrared spectrum of the compound with the mass spectrum shown below has a medium-intensity peak...
Related questions
Question
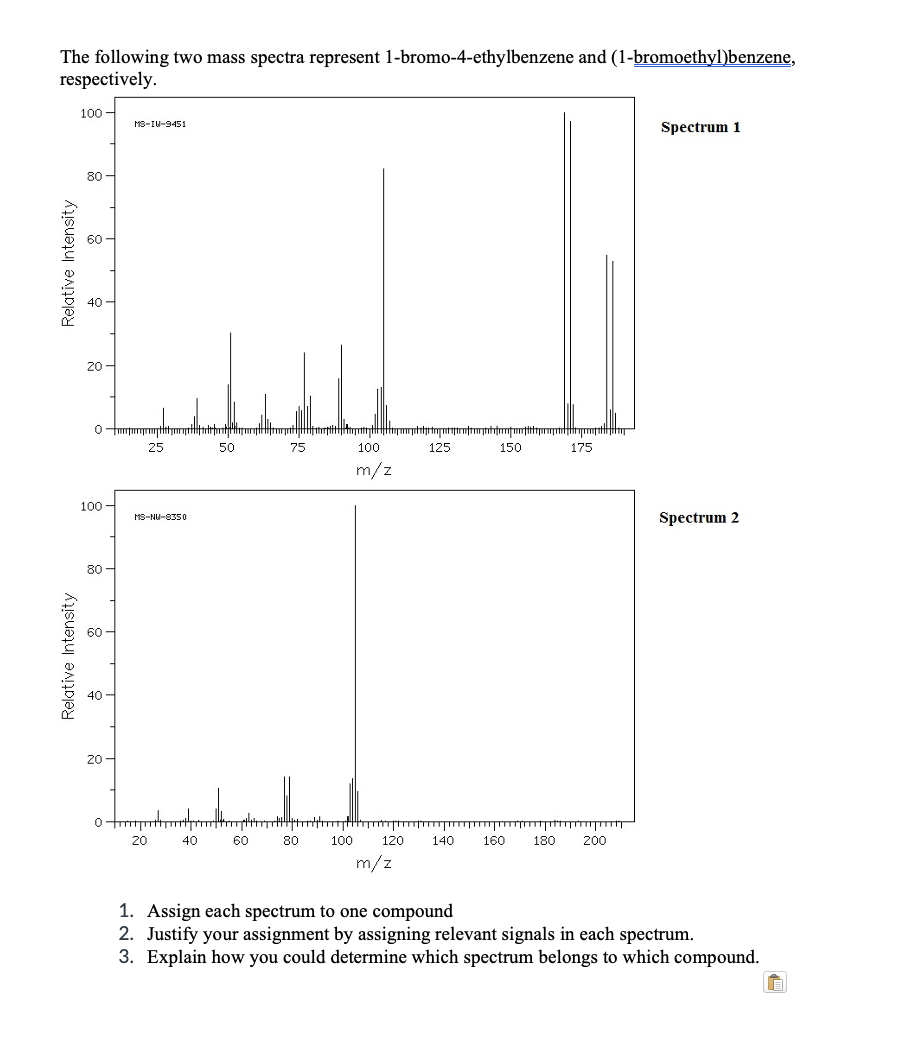
Transcribed Image Text:The following two mass spectra represent 1-bromo-4-ethylbenzene and (1-bromoethyl)benzene,
respectively.
100 -
MS-IU-9451
Spectrum 1
80 -
60
40
20 -
25
50
75
100
125
150
175
m/z
100 -
MS-NU-8350
Spectrum 2
80 -
60
20-
20
40
60
80
100
120
140
160
180
200
m/z
1. Assign each spectrum to one compound
2. Justify your assignment by assigning relevant signals in each spectrum.
3. Explain how you could determine which spectrum belongs to which compound.
Relative Intensity
Relative Intensity
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning