The four bonds of carbon tetrachloride (CCI4) are polar, but the molecule is nonpolar because the bond polarity is canceled by the symmetric tetrahedral shape. When other atoms substitute for some of the Cl atoms, the symmetry is broken and the molecule becomes polar. Use Figure 9.20 to rank the following molecules from the least polar to the most polar: CH2Br2, CF2CI2, CH,F2, CH2CI2, CBr4, CF2B12. (Type your answer using the format NH4 for NH4.) CBr4 < CF2B12 1x < CF2C12 x < CH2B12 CH2C12 x
The four bonds of carbon tetrachloride (CCI4) are polar, but the molecule is nonpolar because the bond polarity is canceled by the symmetric tetrahedral shape. When other atoms substitute for some of the Cl atoms, the symmetry is broken and the molecule becomes polar. Use Figure 9.20 to rank the following molecules from the least polar to the most polar: CH2Br2, CF2CI2, CH,F2, CH2CI2, CBr4, CF2B12. (Type your answer using the format NH4 for NH4.) CBr4 < CF2B12 1x < CF2C12 x < CH2B12 CH2C12 x
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.51QE
Related questions
Question
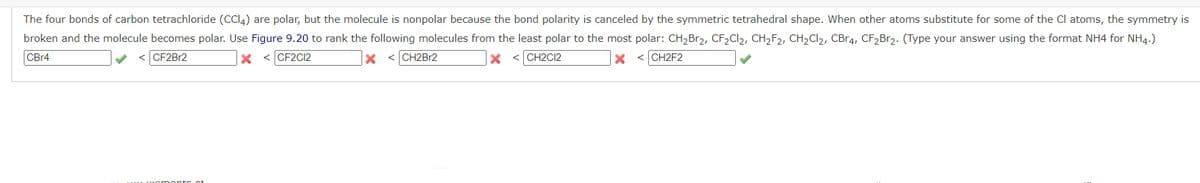
Transcribed Image Text:The four bonds of carbon tetrachloride (CCI4) are polar, but the molecule is nonpolar because the bond polarity is canceled by the symmetric tetrahedral shape. When other atoms substitute for some of the Cl atoms, the symmetry is
broken and the molecule becomes polar. Use Figure 9.20 to rank the following molecules from the least polar to the most polar: CH,Br2, CF2CI2, CH2F2, CH2CI2, CBR4, CF2B12. (Type your answer using the format NH4 for NH4.)
CB14
CF2BR2
x < CF2C2
< CH2B12
< CH2C12
< CH2F2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
