The general values for steric and torsional strain are provided. Use these values to calculate the barrier to rotation for 2-methylpentane looking down the C2-C3 bond. Assume that methyl/ethyl steric interactions are the same as kJ/mol methyl/methyl. 1 2 3 H H. 4 C CH/CH3 gauche `CH3 3.8 kJ/mol steric strain HH 7 8 9 H/H eclipsed bond 4 kJ/mol torsional strain H. H. CH/H eclipsed bond 5.6 kJ/mol torsional strain H H +/- x 10 `CH3 H3C CH3 CH/CH3 eclipsed 11 kJ/mol torsional & steric strain H
The general values for steric and torsional strain are provided. Use these values to calculate the barrier to rotation for 2-methylpentane looking down the C2-C3 bond. Assume that methyl/ethyl steric interactions are the same as kJ/mol methyl/methyl. 1 2 3 H H. 4 C CH/CH3 gauche `CH3 3.8 kJ/mol steric strain HH 7 8 9 H/H eclipsed bond 4 kJ/mol torsional strain H. H. CH/H eclipsed bond 5.6 kJ/mol torsional strain H H +/- x 10 `CH3 H3C CH3 CH/CH3 eclipsed 11 kJ/mol torsional & steric strain H
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.29QAP
Related questions
Question
100%
The general values for steric and torsional strain are provided. Use these values to calculate the barrier to rotation for 2-methylpentane looking down the C2-C3 bond. Assume that methyl/ethyl steric interactions are the same as methyl/methyl.
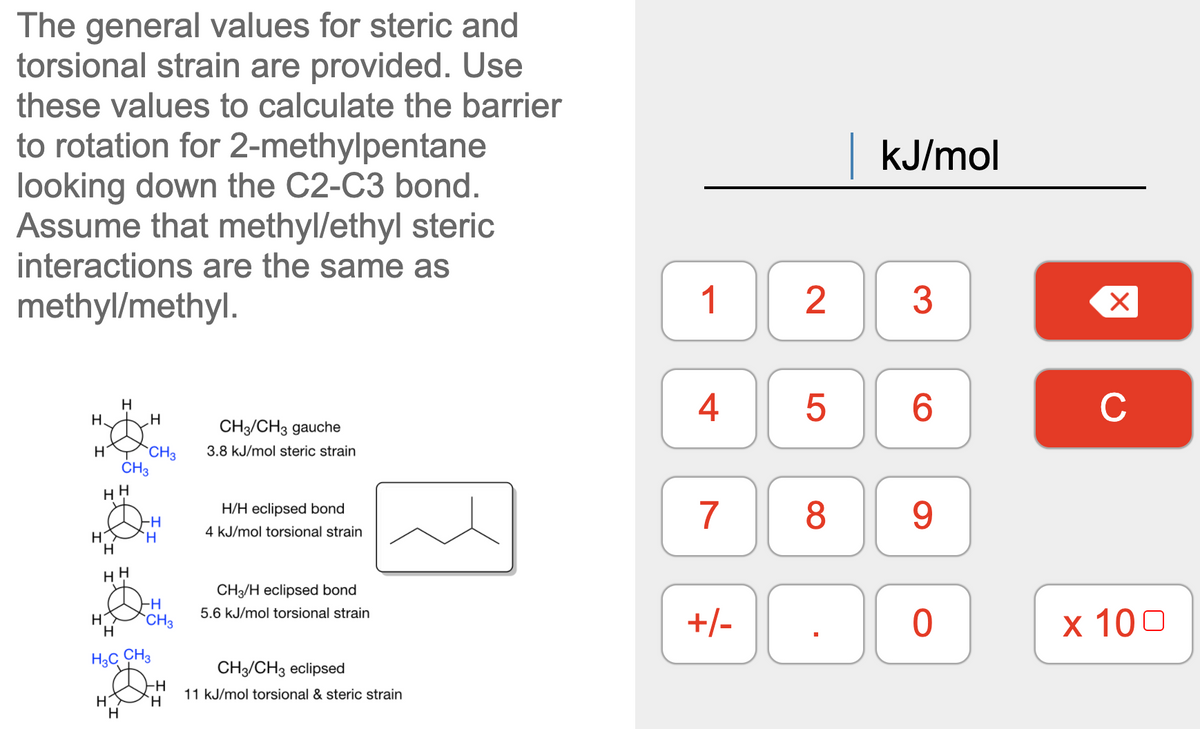
Transcribed Image Text:The general values for steric and
torsional strain are provided. Use
these values to calculate the barrier
to rotation for 2-methylpentane
looking down the C2-C3 bond.
Assume that methyl/ethyl steric
interactions are the same as
kJ/mol
methyl/methyl.
1
2
3
4
6
C
H
H.
.H
CH3/CH3 gauche
`CH3
ČH3
H
3.8 kJ/mol steric strain
7
8
9
H/H eclipsed bond
`H
4 kJ/mol torsional strain
HH
CH3/H eclipsed bond
5.6 kJ/mol torsional strain
+/-
х 100
H
`CH3
H.
H3C CH3
CH/CH3 eclipsed
-H
11 kJ/mol torsional & steric strain
`H
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you



