The half-equivalence point of a titration occurs half way to the equivalence point, where half of the analyte has reacted to form its conjugate, and the other half still remains unreacted. If 0.540 moles of a monoprotic weak acid (Ka = 4.7 × 10-5) is titrated with NaOH, what is the pH of the solution at the half-equivalence point? pH =
The half-equivalence point of a titration occurs half way to the equivalence point, where half of the analyte has reacted to form its conjugate, and the other half still remains unreacted. If 0.540 moles of a monoprotic weak acid (Ka = 4.7 × 10-5) is titrated with NaOH, what is the pH of the solution at the half-equivalence point? pH =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 37QAP: Given three acid-base indicators—methyl orange (end point at pH 4), bromthymol blue (end point at...
Related questions
Question
100%
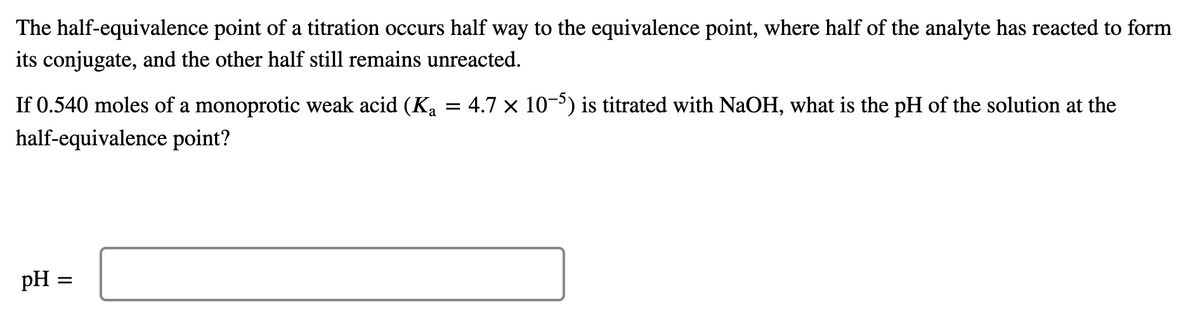
Transcribed Image Text:The half-equivalence point of a titration occurs half way to the equivalence point, where half of the analyte has reacted to form
its conjugate, and the other half still remains unreacted.
If 0.540 moles of a monoprotic weak acid (Ka = 4.7 × 10-5) is titrated with NaOH, what is the pH of the solution at the
half-equivalence point?
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning