The hall ife for the radioactive decay of carbon-14 to nitrogen-14 is 5.73- 10' years. Suppose nucdear chemical analyss shows that there is 0.835 mmol of nitrogen 14 for every 1.000 mmol of carbon-14 in a certan sample of rock. Calutate the age of the rock. Round your answer to 2 signficant digits years
The hall ife for the radioactive decay of carbon-14 to nitrogen-14 is 5.73- 10' years. Suppose nucdear chemical analyss shows that there is 0.835 mmol of nitrogen 14 for every 1.000 mmol of carbon-14 in a certan sample of rock. Calutate the age of the rock. Round your answer to 2 signficant digits years
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 128E: In Active Example 3-29 you calculated that you would have to work six weeks to earn enough money to...
Related questions
Question
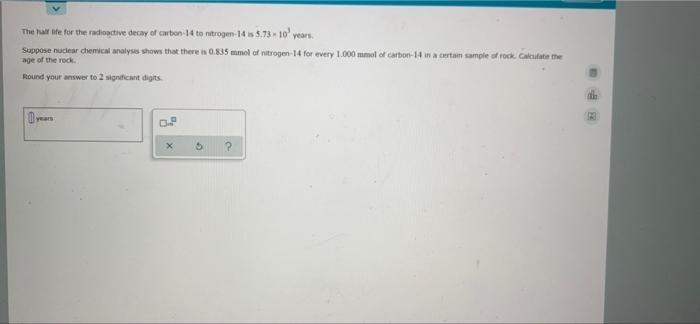
Transcribed Image Text:The hall ife for the radioactive decay of carbon-14 to nitrogen-14 is 5.73- 10' years.
Suppose nuclear chemical analyss shows that there is 0.835 mmol of nitrogen-14 for every 1.000 mmol of carbon-14 in a certam sample of rock. Calculate the
age of the rock.
Round your answer to 2 significant digits
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning