The homogeneity of the chloride level in a water sample from a lake was tested by analyzing por- tions drawn from the top and from near the bottom of the lake, with the following results in ppm CI: Top 26.30 Bottom 26.22 26.43 26.32 26.28 26.20 26.19 26.11 26.49 26.42 (a) Apply the i test at the 95% confidence level to determine if the means are different. (b) Now use the paired / test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level. (c) Why is a different conclusion drawn from using the paired i test than from just pooling the data and using the normal i test for differences in means?
The homogeneity of the chloride level in a water sample from a lake was tested by analyzing por- tions drawn from the top and from near the bottom of the lake, with the following results in ppm CI: Top 26.30 Bottom 26.22 26.43 26.32 26.28 26.20 26.19 26.11 26.49 26.42 (a) Apply the i test at the 95% confidence level to determine if the means are different. (b) Now use the paired / test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level. (c) Why is a different conclusion drawn from using the paired i test than from just pooling the data and using the normal i test for differences in means?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.21QAP
Related questions
Question
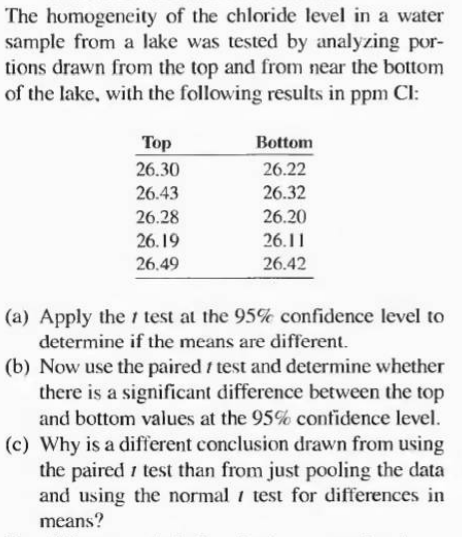
Transcribed Image Text:The homogeneity of the chloride level in a water
sample from a lake was tested by analyzing por-
tions drawn from the top and from near the bottom
of the lake, with the following results in ppm CI:
Top
26.30
Bottom
26.22
26.43
26.32
26.28
26.20
26.19
26.11
26.49
26.42
(a) Apply the i test at the 95% confidence level to
determine if the means are different.
(b) Now use the paired / test and determine whether
there is a significant difference between the top
and bottom values at the 95% confidence level.
(c) Why is a different conclusion drawn from using
the paired i test than from just pooling the data
and using the normal i test for differences in
means?
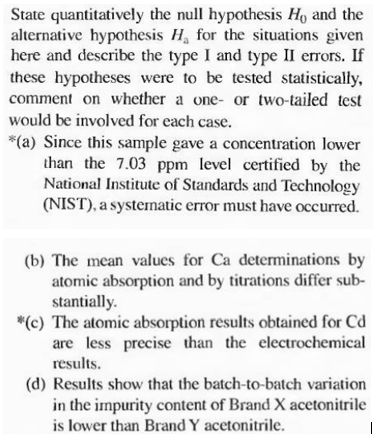
Transcribed Image Text:State quantitatively the null hypothesis Ho and the
alternative hypothesis H, for the situations given
here and describe the type I and type II errors. If
these hypotheses were to be tested statistically,
comment on whether a one- or two-tailed test
would be involved for each case.
*(a) Since this sample gave a concentration lower
than the 7.03 ppm level certified by the
National Institute of Standards and Technology
(NIST), a systematic error must have occurred.
(b) The mean values for Ca determinations by
atomic absorption and by titrations differ sub-
stantially.
*(c) The atomic absorption results obtained for Cd
are less precise than the electrochemical
results.
(d) Results show that the batch-to-batch variation
in the impurity content of Brand X acetonitrile
is lower than Brand Y acetonitrile.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

