The image below shows the different interactions responsible for the spontaneous folding of a protein molecule. Identify which interactions are involved for each labelled region O-H H CH,OH NH, B CH, CH₂COOH H H C CH CH, H--O=C D (CHJ4–NH,
The image below shows the different interactions responsible for the spontaneous folding of a protein molecule. Identify which interactions are involved for each labelled region O-H H CH,OH NH, B CH, CH₂COOH H H C CH CH, H--O=C D (CHJ4–NH,
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter8: Electrochemistry And Ionic Solutions
Section: Chapter Questions
Problem 8.75E: Under what conditions does the extended Debye-Huckel law, equation 8.52, become the Debye-Hckel...
Related questions
Question
please skip if you already answered this. I will upvote if this is typewritten. much appreciated. thank you so much NOTE: THE BIG NUMBER IS FOR NUMBERING ONLY. IT IS NOT GRADED
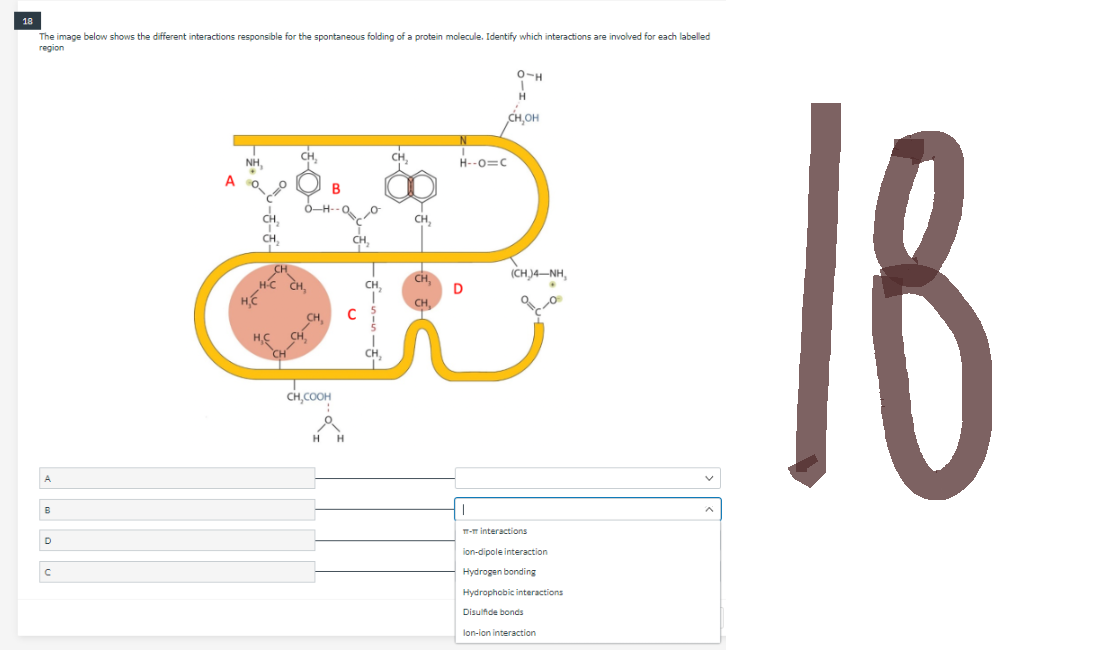
Transcribed Image Text:18
The image below shows the different interactions responsible for the spontaneous folding of a protein molecule. Identify which interactions are involved for each labelled
region
0-H
H
CH,OH
CH,
NH,
0
B
O-H--OO
CH,
CH₂
A
B
D
C
A
यह मह
CH
CH₂COOH
C
H
CH,
CH,
CH,
CH
H--O=C
D
T
(CHJANH,
- interactions
ion-dipole interaction
Hydrogen bonding
Hydrophobic interactions
Disulfide bonds
lon-ion interaction
18
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning