The molar extinction coefficients for A and B at 2=450 nm are ɛ=2,000. and ɛ=3000. M-1cm-1. At =650 nm, the molar extinction coefficients are ɛ=1,000. for both compounds. If a sample containing both species had absorbance values at 2=600 nm are A=0.070 and at 2=400 nm are A=0.185, what concentrations of A and B are present in the unknown solution? Assume that the absorbances are additive and that the measurements were made using a 1-cm cuvette. Show your work.
The molar extinction coefficients for A and B at 2=450 nm are ɛ=2,000. and ɛ=3000. M-1cm-1. At =650 nm, the molar extinction coefficients are ɛ=1,000. for both compounds. If a sample containing both species had absorbance values at 2=600 nm are A=0.070 and at 2=400 nm are A=0.185, what concentrations of A and B are present in the unknown solution? Assume that the absorbances are additive and that the measurements were made using a 1-cm cuvette. Show your work.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.9QAP
Related questions
Question
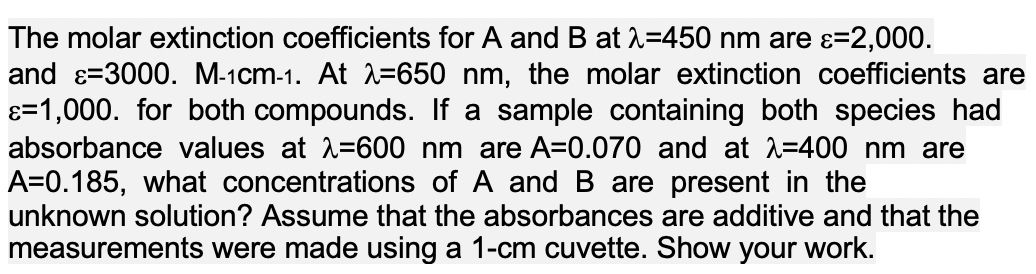
Transcribed Image Text:The molar extinction coefficients for A and B at 2=450 nm are ɛ=2,000.
and ɛ=3000. M-1cm-1. At =650 nm, the molar extinction coefficients are
ɛ=1,000. for both compounds. If a sample containing both species had
absorbance values at 2=600 nm are A=0.070 and at 2=400 nm are
A=0.185, what concentrations of A and B are present in the
unknown solution? Assume that the absorbances are additive and that the
measurements were made using a 1-cm cuvette. Show your work.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning