Beer's Law Plot for Tartrazine Concentration Absorbance Linear Fit for: Data Set | Absorbance (µM or umol/L) Abs = mx+b m (Slope): 0.02534 +/- 0.0003590 0.001 0.00 b (Y-Intercept): -0.0009147 +/- 0.008746 Correlation: 0.9997 0.252 9.94 RMSE: 0.01129 0.5 19.9 0.506 29.8 0.738 39.8 1.018 10 30 40 20 Concentration (uM) slope (m): 0.02534 1/(µM) y-intercept (b): -0.0009147 Absorbance
Beer's Law Plot for Tartrazine Concentration Absorbance Linear Fit for: Data Set | Absorbance (µM or umol/L) Abs = mx+b m (Slope): 0.02534 +/- 0.0003590 0.001 0.00 b (Y-Intercept): -0.0009147 +/- 0.008746 Correlation: 0.9997 0.252 9.94 RMSE: 0.01129 0.5 19.9 0.506 29.8 0.738 39.8 1.018 10 30 40 20 Concentration (uM) slope (m): 0.02534 1/(µM) y-intercept (b): -0.0009147 Absorbance
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.12QAP
Related questions
Question
1) Calculate the molar extinction coefficient (molar absorptivity) of tartrazine in units of 1/(M·cm). Assume a cuvette path length of 1.00 cm.
2) If Ana determines the absorbance of an unknown tartrazine sample to be 0.662, what is the concentration of the sample in µM?
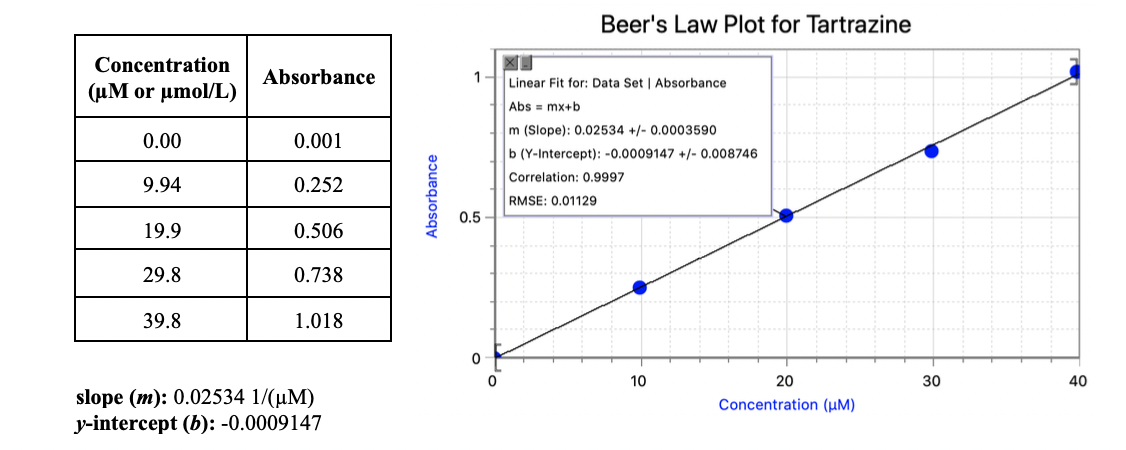
Transcribed Image Text:Beer's Law Plot for Tartrazine
Concentration
Absorbance
Linear Fit for: Data Set | Absorbance
(µM or umol/L)
Abs = mx+b
m (Slope): 0.02534 +/- 0.0003590
0.001
0.00
b (Y-Intercept): -0.0009147 +/- 0.008746
Correlation: 0.9997
0.252
9.94
RMSE: 0.01129
0.5
19.9
0.506
29.8
0.738
39.8
1.018
10
30
40
20
Concentration (uM)
slope (m): 0.02534 1/(µM)
y-intercept (b): -0.0009147
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

