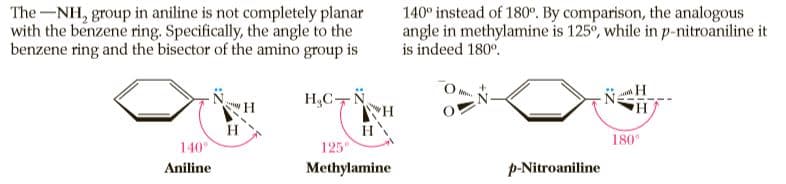
The-NH, group in aniline is not completely planar with the benzene ring. Specifically, the angle to the benzene ring and the bisector of the amino group is 140° instead of 180°. By comparison, the analogous angle in methylamine is 125°, while in p-nitroaniline it is indeed 180°. H,C-N H HA H 140° 180 125 Aniline Methylamine p-Nitroaniline
Coding Strand of DNA
When pointing to DNA transcription, the coding strand is found to be the DNA strand whose base sequence is indistinguishable from the base sequence of the RNA transcript developed. It is this strand that comprises the codons, while the non-coding strand comprises the anti-codons.
Nucleotide
Both DNA and RNA are composed of organic molecules known as nucleotides. Hence, nucleotides are known as the basic building blocks of nucleic acids. These substances play a role in various processes such as cell signalling, enzyme reactions, metabolism, and so on.
Structure of Cytosine
Cytosine is among the five primary nitrogenous bases of which DNA and RNA and are being used in storage and transportation of genetic makeup within a cell. Adenine, guanine, thymine as well as uracil are the remaining four nucleobases.
In the structures of T—A and C—G base pairs, there are three amino groups specifically labeled as “sp2 hybridized and planar”. What is the primary
difference between these structures and that of
aniline that lead to their planarity?
1. In contrast to aniline, the amino groups on the DNA bases are necessary to make the heterocyclic rings
2. In contrast to aniline, the contributing structures that delocalize the nitrogen lone pairs onto the rings creates partial negative charges on electronegative atoms.
3. In contrast to aniline, the hydrogen bond accepting ability of the lone pairs on the -NH2
groups of the DNA bases is better when these amino groups are sp2 hybridized.
4. Both 2 and 3.
Step by step
Solved in 3 steps with 1 images







