The normal boiling point of a certain liquid X is 136.50 °C, but when 20. g of iron(III) nitrate (Fe(NO3)3) a 138.2 °C instead. Use this information to calculate the molal boiling point elevation constant K, of X. Round your answer to 2 significant digits. are dissolved in 100. g of X the solution boils at
The normal boiling point of a certain liquid X is 136.50 °C, but when 20. g of iron(III) nitrate (Fe(NO3)3) a 138.2 °C instead. Use this information to calculate the molal boiling point elevation constant K, of X. Round your answer to 2 significant digits. are dissolved in 100. g of X the solution boils at
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.103QE
Related questions
Question
Help with the following question
Round the answer to 2 sig figs
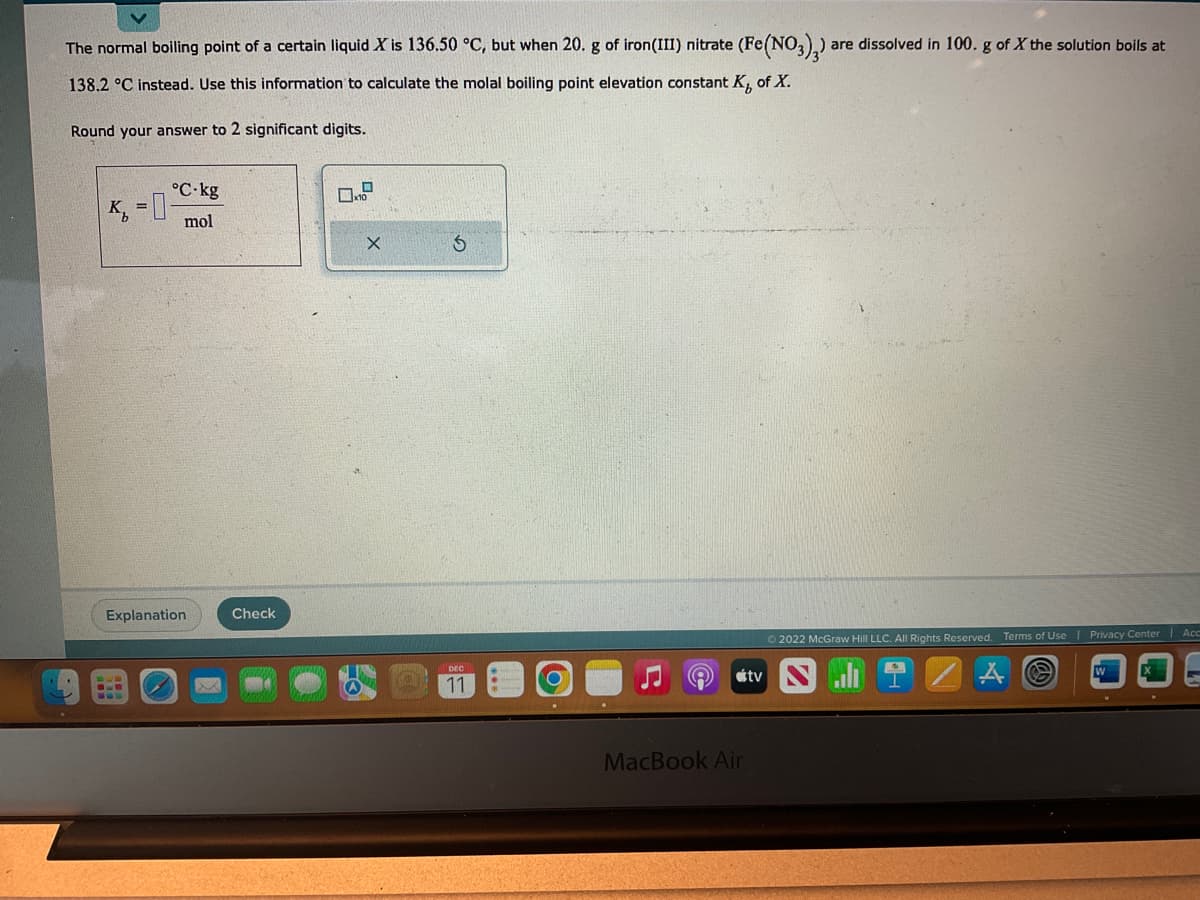
Transcribed Image Text:The normal boiling point of a certain liquid X is 136.50 °C, but when 20. g of iron(III) nitrate (Fe(NO3)2) are dissolved in 100. g of X the solution boils at
138.2 °C instead. Use this information to calculate the molal boiling point elevation constant K, of X.
Round your answer to 2 significant digits.
°C.kg
K₁ = -
www
mol
Explanation.
Check
D
X
S
DEC
11
O
MacBook Air
tv
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acc
A
W
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning