The number of electrons that each energy level n can hold is set by the possible quantum numbers and the Pauli Exclusion Principle. Since only one electron can occupy a given quantum state, you can only have as many electrons as quantum states (n, I, mı, m, combinations). Because m, can always be 1/2 (spin up) or -1/2 (spin down) regardless of the n, I, m¡ combination, all you need to do is to find how many possible I, m, combinations exist for a given n and multiply by 2 in order to find the total number of states for a given n. For n = 1: | = 0 and m = 0, thus at the first shell (ground state) you can only have 1 1, m, combination, or 2*(1) electrons. For n = 2: | = 0, 1 (0 or 1) when I = 0: m = 0 when I = 1: m, = -1, 0, 1 (-1 or O or 1), thus at the second shell you can have 3+1 I, m¡ combinations, or 2*(4) electrons. How many electrons can you have on the 3rd shell (n = 3)? Hopefully by working out the answer of the previous question you realized that there is a pattern for the number of allowed states at each energy level. This pattern can be represented by a simple formula which can be used to quickly tell the number of electrons that a given shell can hold. The formula is 2*n? How many electrons can the n = 4 shell hold?
The number of electrons that each energy level n can hold is set by the possible quantum numbers and the Pauli Exclusion Principle. Since only one electron can occupy a given quantum state, you can only have as many electrons as quantum states (n, I, mı, m, combinations). Because m, can always be 1/2 (spin up) or -1/2 (spin down) regardless of the n, I, m¡ combination, all you need to do is to find how many possible I, m, combinations exist for a given n and multiply by 2 in order to find the total number of states for a given n. For n = 1: | = 0 and m = 0, thus at the first shell (ground state) you can only have 1 1, m, combination, or 2*(1) electrons. For n = 2: | = 0, 1 (0 or 1) when I = 0: m = 0 when I = 1: m, = -1, 0, 1 (-1 or O or 1), thus at the second shell you can have 3+1 I, m¡ combinations, or 2*(4) electrons. How many electrons can you have on the 3rd shell (n = 3)? Hopefully by working out the answer of the previous question you realized that there is a pattern for the number of allowed states at each energy level. This pattern can be represented by a simple formula which can be used to quickly tell the number of electrons that a given shell can hold. The formula is 2*n? How many electrons can the n = 4 shell hold?
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter8: Quantum Mechanics In Three Dimensions
Section: Chapter Questions
Problem 20P
Related questions
Question
100%
Please help with both parts of the question. Thank you in advance!
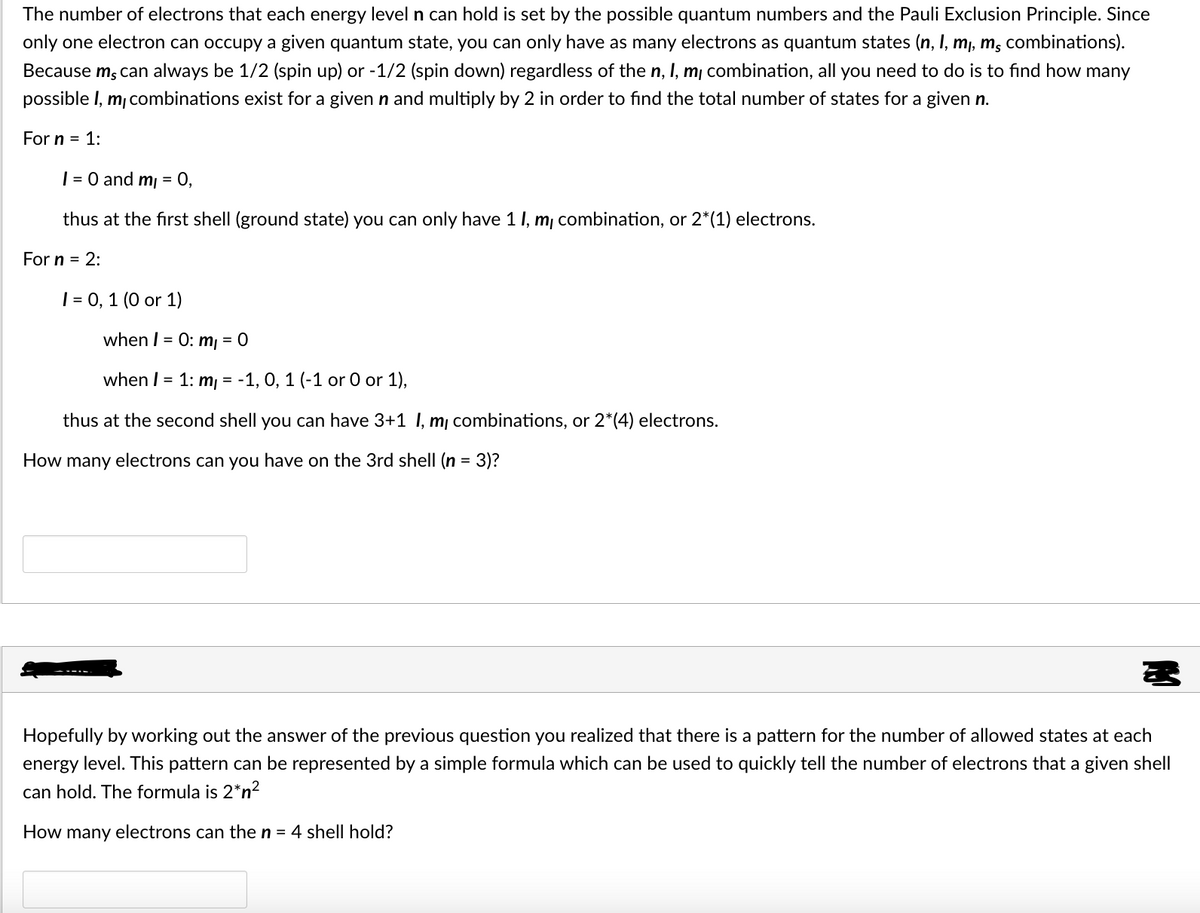
Transcribed Image Text:The number of electrons that each energy level n can hold is set by the possible quantum numbers and the Pauli Exclusion Principle. Since
only one electron can occupy a given quantum state, you can only have as many electrons as quantum states (n, I, m, m, combinations).
Because m, can always be 1/2 (spin up) or -1/2 (spin down) regardless of the n, I, mj combination, all you need to do is to find how many
possible I, m, combinations exist for a given n and multiply by 2 in order to find the total number of states for a given n.
For n = 1:
| = 0 and mi
0,
thus at the first shell (ground state) you can only have 1 1, m, combination, or 2*(1) electrons.
For n = 2:
| = 0, 1 (0 or 1)
when I = 0: m = 0
when I = 1: m, = -1, 0, 1 (-1 or 0 or 1),
thus at the second shell you can have 3+1 I, m combinations, or 2*(4) electrons.
How many electrons can you have on the 3rd shell (n = 3)?
Hopefully by working out the answer of the previous question you realized that there is a pattern for the number of allowed states at each
energy level. This pattern can be represented by a simple formula which can be used to quickly tell the number of electrons that a given shell
can hold. The formula is 2*n?
How many electrons can the n = 4 shell hold?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax