The octanol - water aqueous buffer partition coefficients (P) for a drug used to treat glaucoma were obtained experimentally at various temperatures (T) and pH values. The results are presented in the following table: pH=6.25 pH=7.0 pH=7.25 T= 27 C 0.24 0.72 0.89 T= 30 C 0.31 0.84 1.06 T= 40 C 1.23 27.4 mg of this drug were dissolved in 100 mL of buffered water (pH=7.0; T=30) and then partitioned with octanol portions of 37.1 mL. Calculate how many mg of drug will remain in water after in the first extraction (Fill in ONLY numbers approximated to the 2nd decimal place) 1.49 Answer: 19.01
The octanol - water aqueous buffer partition coefficients (P) for a drug used to treat glaucoma were obtained experimentally at various temperatures (T) and pH values. The results are presented in the following table: pH=6.25 pH=7.0 pH=7.25 T= 27 C 0.24 0.72 0.89 T= 30 C 0.31 0.84 1.06 T= 40 C 1.23 27.4 mg of this drug were dissolved in 100 mL of buffered water (pH=7.0; T=30) and then partitioned with octanol portions of 37.1 mL. Calculate how many mg of drug will remain in water after in the first extraction (Fill in ONLY numbers approximated to the 2nd decimal place) 1.49 Answer: 19.01
Chapter14: Chromatography
Section: Chapter Questions
Problem 10P
Related questions
Question
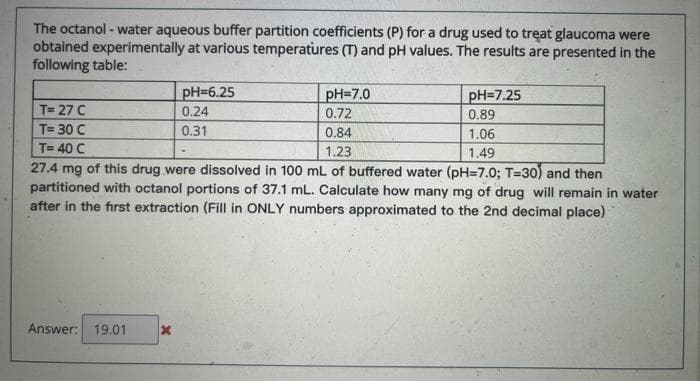
Transcribed Image Text:The octanol - water aqueous buffer partition coefficients (P) for a drug used to treat glaucoma were
obtained experimentally at various temperatures (T) and pH values. The results are presented in the
following table:
pH=7.0
0.72
0.84
pH=6.25
pH=7.25
T= 27 C
0.24
0.89
T= 30 C
0.31
1.06
T= 40 C
1.23
1.49
27.4 mg of this drug were dissolved in 100 mL of buffered water (pH=7.0; T=30) and then
partitioned with octanol portions of 37.1 mL. Calculate how many mg of drug will remain in water
after in the first extraction (Fill in ONLY numbers approximated to the 2nd decimal place)
Answer: 19.01
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning