The organic compound trishydroxymethylaminomethane is abbreviated Tris and is basic. The conjugate acid (TrisH+) has a pKa of 8.10. TrisH++ H20 = Tris + H30+ Tris is commonly used as a component in buffer solutions to study biochemical reactions. Part A A buffer solution is prepared by mixing 12.5 mL of 0.100 mol L-'HCI with 25 mL of 0.100 mol L-'Tris. What the pH of this buffer? Express your answer using two decimal places. Templates Symbols uado redo reset keyboard shortcuts help pH = Submit Request Answer • Part B The buffer was used to study an enzyme-catalyzed reaction in which 0.00030 mol of H30+ was produced. What is the pH of the solution after the acid reacted with the buffer? Express your answer using two decimal places. Templates Symbols uado redo reset keyboard shortcuts help, pH =
The organic compound trishydroxymethylaminomethane is abbreviated Tris and is basic. The conjugate acid (TrisH+) has a pKa of 8.10. TrisH++ H20 = Tris + H30+ Tris is commonly used as a component in buffer solutions to study biochemical reactions. Part A A buffer solution is prepared by mixing 12.5 mL of 0.100 mol L-'HCI with 25 mL of 0.100 mol L-'Tris. What the pH of this buffer? Express your answer using two decimal places. Templates Symbols uado redo reset keyboard shortcuts help pH = Submit Request Answer • Part B The buffer was used to study an enzyme-catalyzed reaction in which 0.00030 mol of H30+ was produced. What is the pH of the solution after the acid reacted with the buffer? Express your answer using two decimal places. Templates Symbols uado redo reset keyboard shortcuts help, pH =
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 2ALQ: A friend asks the following: Consider a buffered solution made up of the weak acid HA and its salt...
Related questions
Question
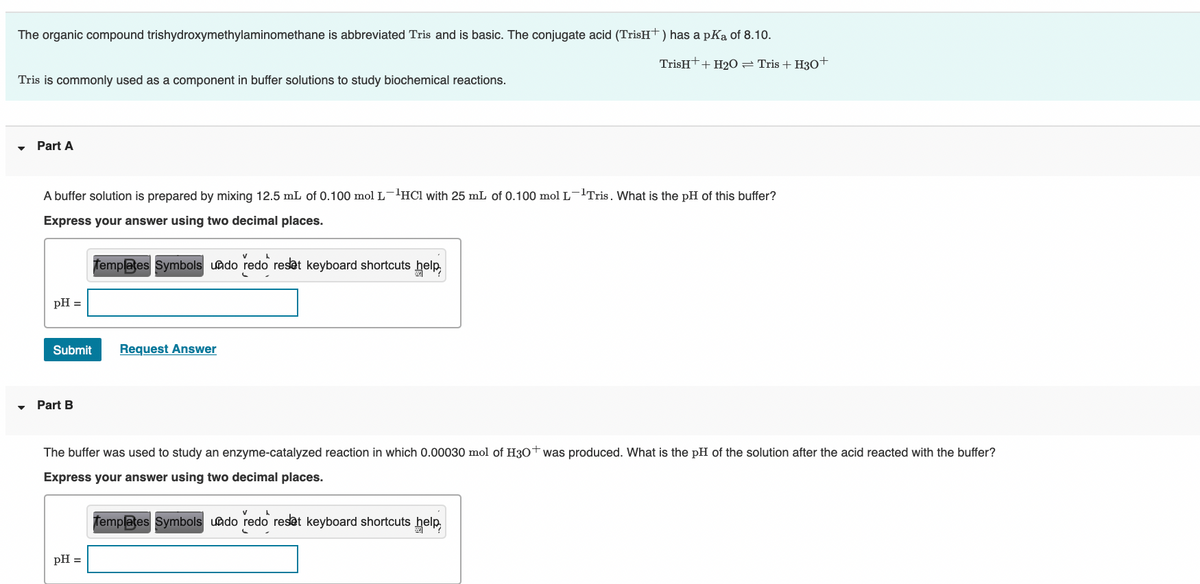
Transcribed Image Text:The organic compound trishydroxymethylaminomethane is abbreviated Tris and is basic. The conjugate acid (TrisH+) has a pKa of 8.10.
TrisHt+ H20= Tris + H30+
Tris is commonly used as a component in buffer solutions to study biochemical reactions.
Part A
A buffer solution is prepared by mixing 12.5 mL of 0.100 mol L'HCl with 25 mL of 0.100 mol L-lTris. What is the pH of this buffer?
Express your answer using two decimal places.
femplates Symbols uado redo reset keyboard shortcuts help,
pH =
Submit
Request Answer
Part B
The buffer was used to study an enzyme-catalyzed reaction in which 0.00030 mol of H30+was produced. What is the pH of the solution after the acid reacted with the buffer?
Express your answer using two decimal places.
Templates Symbols uado redo reset keyboard shortcuts help
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning