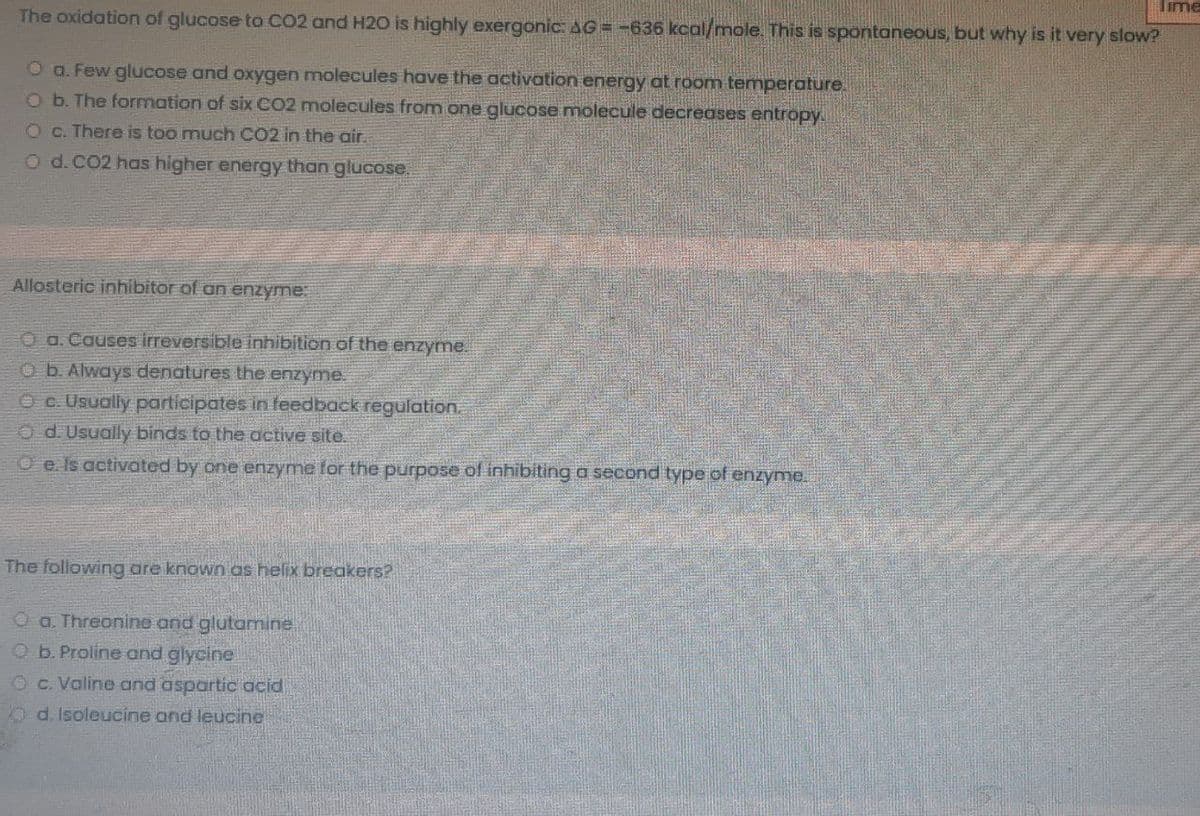
The oxidation of glucose to CO2 and H2O is highly exergonic: AG= -636 kcal/mole. This is spontaneous, b O a. Few glucose and oxygen molecules have the activation energy at room temperature. ob. The formation of six CO2 molecules from one glucose molecule decreases entropy. Oc. There is too much CO2 in the air. Od.CO2 has higher energy than glucose. Allosteric inhibitor of an enzyme O a. Causes irreversible inhibition of the enzyme. O b. Always denatures the enzyme. Oc. Usually participates in feedback regulation. O d. Usually binds to the active site. O e. Is activated by one enzyme tor the purpose of inhibiting a second type of enzyme. The following are known as helix breakers? O a. Threonine and glutamine Ob. Proline and glycine O c. Valine and aspartic acid d. Isoleucine and leucine
The oxidation of glucose to CO2 and H2O is highly exergonic: AG= -636 kcal/mole. This is spontaneous, b O a. Few glucose and oxygen molecules have the activation energy at room temperature. ob. The formation of six CO2 molecules from one glucose molecule decreases entropy. Oc. There is too much CO2 in the air. Od.CO2 has higher energy than glucose. Allosteric inhibitor of an enzyme O a. Causes irreversible inhibition of the enzyme. O b. Always denatures the enzyme. Oc. Usually participates in feedback regulation. O d. Usually binds to the active site. O e. Is activated by one enzyme tor the purpose of inhibiting a second type of enzyme. The following are known as helix breakers? O a. Threonine and glutamine Ob. Proline and glycine O c. Valine and aspartic acid d. Isoleucine and leucine
Biochemistry
6th Edition
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Reginald H. Garrett, Charles M. Grisham
Chapter23: Fatty Acid Catabolism
Section: Chapter Questions
Problem 12P: Understanding Human Energy Consumption During Exercise Energy production in animals is related to...
Related questions
Question
I need the answer as soon as possible
Transcribed Image Text:Time
The oxidation of glucose to CO2 and H20 is highly exergonic: AG = -636 kcal/mole. This is spontaneous, but why is it very slow?
O a. Few glucose and oxygen molecules have the activation energy at room temperature.
Ob. The formation of six CO2 molecules from one glucose molecule decreases entropy.
Oc. There is too much CO2 in the air.
O d.CO2 has higher energy than glucose.
Allosteric inhibitor of an enzyme:
O a.Causes irreversible inhibition of the enzyme.
Ob. Always denatures the enzyme.
O c. Usuolly participates in feedback regulation.
O d. Usually binds to the active site.
O e. Is activated by one enzyme for the purpose of inhibiting a second type of enzyme.
The following are known as helix breakers?
O a. Threonine and glutamine
Ob. Proline and glycine
Oc. Valine and aspartic acid
O d. Isoleucine and leucine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning


Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning
