The partial pressure of CO, in air is approximately 4.0 10 4 3.2 x 10 mol/(L-atm) to find the concentration of CO, in atm. If the Henry's-law constant is Part A A can of soda under a CO, pressure of 24 atm at 25 "C Express your answer to two significant figures and include the appropriate units. MA Value Units Enter your answer using unts of chemical concentration No credit lost. Tty again Submit Ereis Answers Request Answer The partial pressure of CO, in air is approximately 4.0 10 atm. If the Henry's-law constant is 3.2 x 10 mol/(L atm) to find the concentration of CO, in Enter your answer using unts of chemical concentration No credit lost Try again Submit Previous Answern Request Answr Part B A can of soda open to the atmosphere at 25 "C Express your answer to two significant figures and include the appropriate units Value Units Submit RestAnser
The partial pressure of CO, in air is approximately 4.0 10 4 3.2 x 10 mol/(L-atm) to find the concentration of CO, in atm. If the Henry's-law constant is Part A A can of soda under a CO, pressure of 24 atm at 25 "C Express your answer to two significant figures and include the appropriate units. MA Value Units Enter your answer using unts of chemical concentration No credit lost. Tty again Submit Ereis Answers Request Answer The partial pressure of CO, in air is approximately 4.0 10 atm. If the Henry's-law constant is 3.2 x 10 mol/(L atm) to find the concentration of CO, in Enter your answer using unts of chemical concentration No credit lost Try again Submit Previous Answern Request Answr Part B A can of soda open to the atmosphere at 25 "C Express your answer to two significant figures and include the appropriate units Value Units Submit RestAnser
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter10: Solutions
Section: Chapter Questions
Problem 66QAP: The Henry's law constant for the solubility of radon in water at is 9.57106 M/mm Hg. Radon is...
Related questions
Question
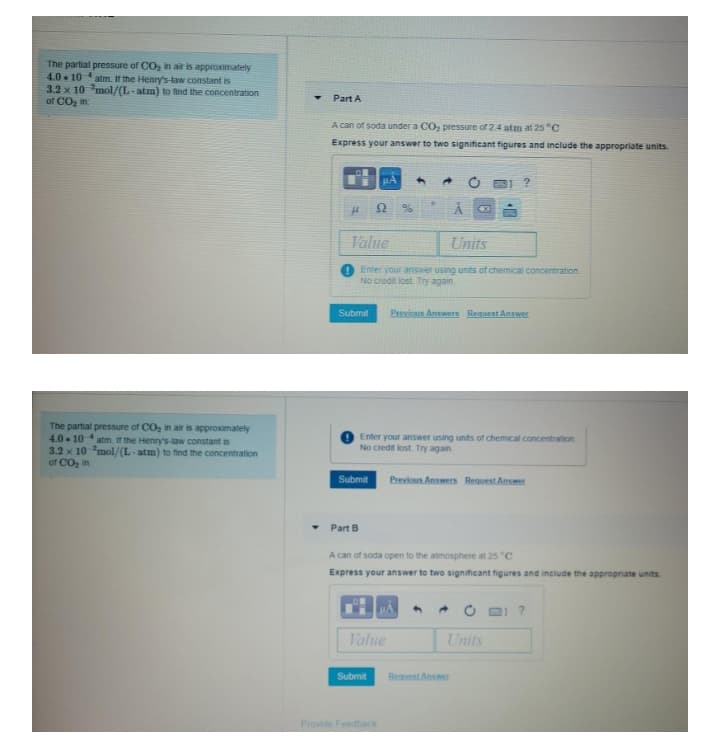
Transcribed Image Text:The partial pressure of CO, in air is approximately
4.0 10 atm. If the Henry's-law constant is
3.2 x 10 mol/(L- atm) to find the concentration
of CO, in
Part A
A can of soda under a CO, pressure of 24 atm at 25 "C
Express your answer to two significant figures and include the appropriate units.
HA
Value
Units
Enter your answer using units of chemical concentration.
No credit lost. Try again
Submit
Previous Answers Request Answer
The partial pressure of CO, in air is approximately
4.0 . 10 atm. If the Henry's-law constant is
3.2 x 10 mol/(L- atm) to find the concentration
of CO, in
Enter your answer using units of chemical concentration
No credit lost. Try again
Submit
Previous Answers Request Answer
Part B
A can of soda open to the atmosphere at 25 "C
Express your answer to two significant figures and include the appropriate units.
Value
Units
Submit
Request Answer
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning