The pka values of OH group in 4-cyanophenol and 4-nitrophenol are identical BUT in the di-methylswbstituted compounds C and D differ significantly. The cyano derivative C is more acidic than compound D. Please briefly explain why this is the case (you can use words and drawings). Hint: delocalization... A В OH OH OH OH CN NO2 CN NO2
The pka values of OH group in 4-cyanophenol and 4-nitrophenol are identical BUT in the di-methylswbstituted compounds C and D differ significantly. The cyano derivative C is more acidic than compound D. Please briefly explain why this is the case (you can use words and drawings). Hint: delocalization... A В OH OH OH OH CN NO2 CN NO2
Chapter22: Carbonyl Alpha-substitution Reactions
Section22.SE: Something Extra
Problem 18VC
Related questions
Question
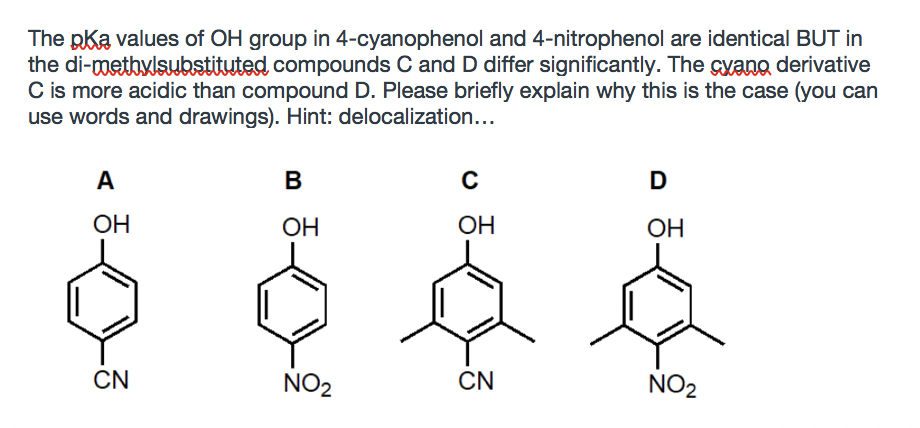
Transcribed Image Text:The pka values of OH group in 4-cyanophenol and 4-nitrophenol are identical BUT in
the di-metbylsbstituted compounds C and D differ significantly. The cyano derivative
C is more acidic than compound D. Please briefly explain why this is the case (you can
use words and drawings). Hint: delocalization...
A
B
ОН
OH
OH
ОН
CN
NO2
CN
NO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



