The position of the phases in the separatory funnel (whether the top or bottom phase) is dependent on the relative densities of the solvents. Complete table 1 to indicate whether each of the solvents will be on the top or bottom layer. Table 1. Solubility properties of common organic solvents. Liquid Solubility in H2O Solubility of H20 (g/L, 20-25°C) 9.5 × 10-3 1.79 Phase layer (g/L, 20-25°C) 0.31 Density (g/mL, 25°C) 0.661 hexane 0.76 0.87 0.877 1.489 benzene chloroform 8.09
The position of the phases in the separatory funnel (whether the top or bottom phase) is dependent on the relative densities of the solvents. Complete table 1 to indicate whether each of the solvents will be on the top or bottom layer. Table 1. Solubility properties of common organic solvents. Liquid Solubility in H2O Solubility of H20 (g/L, 20-25°C) 9.5 × 10-3 1.79 Phase layer (g/L, 20-25°C) 0.31 Density (g/mL, 25°C) 0.661 hexane 0.76 0.87 0.877 1.489 benzene chloroform 8.09
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter9: Liquids, Solids, And Materials
Section: Chapter Questions
Problem 104QRT: Consider this information regarding two compounds. Thallium azide: yellow crystalline solid; melting...
Related questions
Question
100%
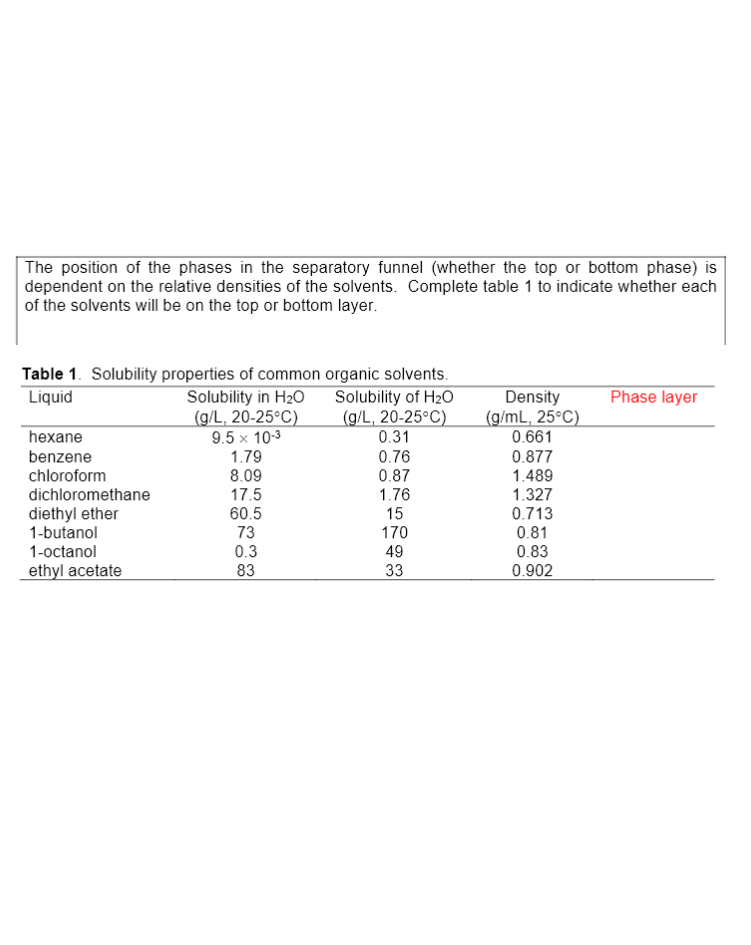
Transcribed Image Text:The position of the phases in the separatory funnel (whether the top or bottom phase) is
dependent on the relative densities of the solvents. Complete table 1 to indicate whether each
of the solvents will be on the top or bottom layer.
Table 1. Solubility properties of common organic solvents.
Solubility in H20
(g/L, 20-25°C)
9.5 x 10-3
1.79
8.09
17.5
Solubility of H20
(g/L, 20-25°C)
0.31
Phase layer
Density
(g/mL, 25°C)
0.661
Liquid
hexane
benzene
chloroform
0.76
0.87
1.76
15
170
0.877
dichloromethane
diethyl ether
1-butanol
60.5
73
0.3
1.489
1.327
0.713
0.81
0.83
1-octanol
49
ethyl acetate
83
33
0.902
Expert Solution
Introduction
“Since there are multiple sub-parts in this question and it is not mentioned that which one has to be solved so I am solving only the first three sub-parts. Please repost the other sub-parts if you need answers for them too.
Separating funnel is ude to separate a mixture of components of different densities.
Most commonly it is used for the separation of organic and aqeous layers.
Those compounds which are more denser will be at the bottom phase.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole