The preexponential and activation energy for the diffusion of iron in cobalt are 1.1 x 105 m²/s and 253,300 J/mol, respectively. At what temperature will the diffusion coefficient have a value of 2.1 x 10 4 m/s?
The preexponential and activation energy for the diffusion of iron in cobalt are 1.1 x 105 m²/s and 253,300 J/mol, respectively. At what temperature will the diffusion coefficient have a value of 2.1 x 10 4 m/s?
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 86AP: The mean free path for methane at a temperature of 269 K and a pressure of 1.11105 Pa is 4.81108 m....
Related questions
Question
I need the answer as soon as possible
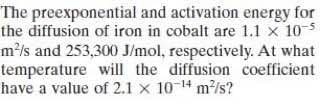
Transcribed Image Text:The preexponential and activation energy for
the diffusion of iron in cobalt are 1.1 x 105
m²/s and 253,300 J/mol, respectively. At what
temperature will the diffusion coefficient
have a value of 2.1 x 10-14 m/s?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University


University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University