The total translational kinetic energy of the molecules of a sample of gas at 415 K is 17500 J. How many moles n does the sample comprise? n = mol Find the average translational kinetic energy K, of a single molecule. Ky = J
The total translational kinetic energy of the molecules of a sample of gas at 415 K is 17500 J. How many moles n does the sample comprise? n = mol Find the average translational kinetic energy K, of a single molecule. Ky = J
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Katz, Debora M.
Chapter20: Kinetic Theory Of Gases
Section: Chapter Questions
Problem 61PQ
Related questions
Question
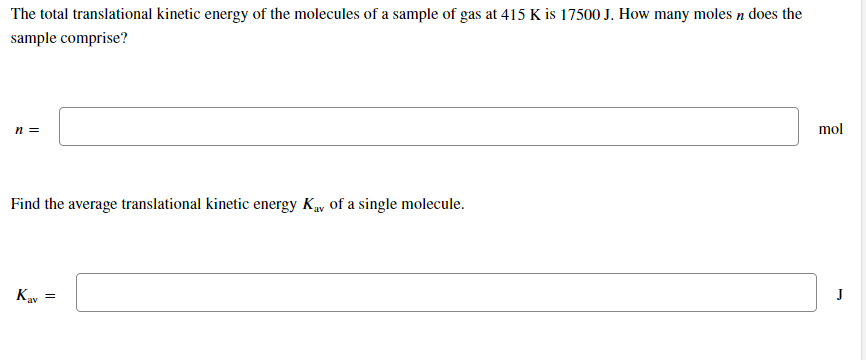
Transcribed Image Text:The total translational kinetic energy of the molecules of a sample of gas at 415 K is 17500 J. How many moles n does the
sample comprise?
mol
n =
Find the average translational kinetic energy Ky of a single molecule.
J
Kav
Expert Solution
Step 1
Given that
The total Translational kinetic energy(K.E) = 17500J
Temperature (T) = 415K
According to the kinetic theory of the gases, The translational Kinetic energy of the molecules is given by
Where n is the number of the moles and R is a gas constant ( )
putting all the given values in the above equation, we get
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning