The protonated form of the R group of lysine is shown in the structure. The ratio of the charged (protonated) form to the deprotonated form depends on the pK, of the R group and the pH of the solution. Select all the pH values at which the charged form of the R group would predominate. 12.0 10.5 5.4 2.2
The protonated form of the R group of lysine is shown in the structure. The ratio of the charged (protonated) form to the deprotonated form depends on the pK, of the R group and the pH of the solution. Select all the pH values at which the charged form of the R group would predominate. 12.0 10.5 5.4 2.2
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter21: Biochemistry
Section: Chapter Questions
Problem 10A
Related questions
Question
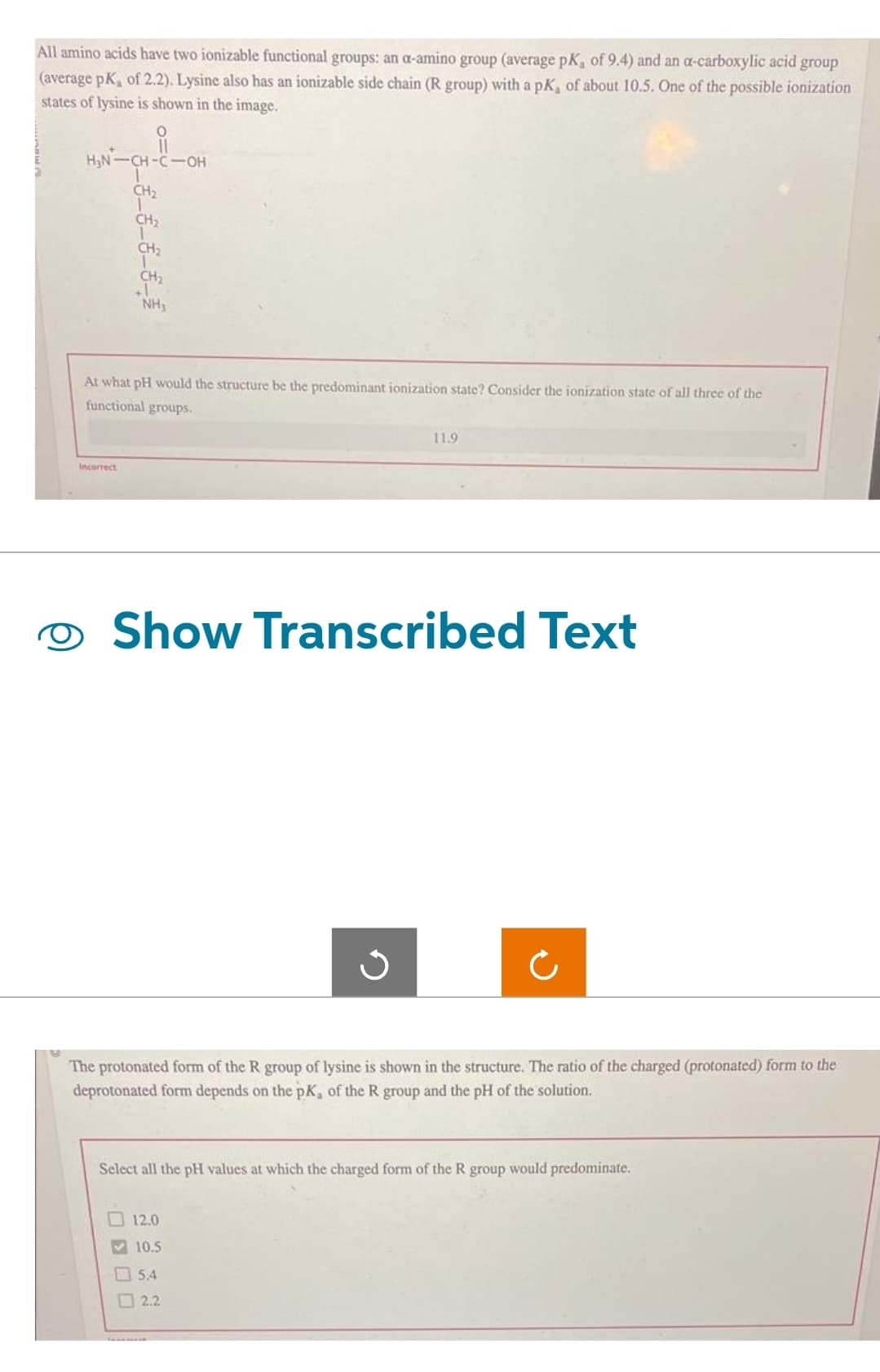
Transcribed Image Text:All amino acids have two ionizable functional groups: an a-amino group (average pK, of 9.4) and an a-carboxylic acid group
(average pK, of 2.2). Lysine also has an ionizable side chain (R group) with a pK, of about 10.5. One of the possible ionization
states of lysine is shown in the image.
i
H₂N-CH-C-OH
CH₂
1
Incorrect
CH₂
CH₂
CH₂
NH₂
At what pH would the structure be the predominant ionization state? Consider the ionization state of all three of the
functional groups.
Show Transcribed Text
11.9
S
The protonated form of the R group of lysine is shown in the structure. The ratio of the charged (protonated) form to the
deprotonated form depends on the pK, of the R group and the pH of the solution.
12.0
10.5
5.4
Select all the pH values at which the charged form of the R group would predominate.
2.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,