The quantum yield of light-induced chemical reactions (called photochemical reactions) measures the efficiency of the process. The quantum yield, d, is defined 4, as: number of reaction events number of photons absorbed Suppose the quantum yield for the reaction CH;X - CH3 + X is =0.24. A cuvette con- taining a solution of CH3X is irradiated with 280-nm light with a power of 885 mW for 10.0 minutes. Assuming total absorp- tion of the light by the sample, what is the maximum amount (in moles) of CH3X that breaks apart?
The quantum yield of light-induced chemical reactions (called photochemical reactions) measures the efficiency of the process. The quantum yield, d, is defined 4, as: number of reaction events number of photons absorbed Suppose the quantum yield for the reaction CH;X - CH3 + X is =0.24. A cuvette con- taining a solution of CH3X is irradiated with 280-nm light with a power of 885 mW for 10.0 minutes. Assuming total absorp- tion of the light by the sample, what is the maximum amount (in moles) of CH3X that breaks apart?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter8: An Introduction To Optical Atomic Spectrometry
Section: Chapter Questions
Problem 8.9QAP
Related questions
Question
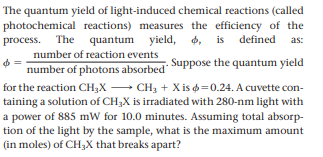
Transcribed Image Text:The quantum yield of light-induced chemical reactions (called
photochemical reactions) measures the efficiency of the
process. The quantum yield,
d, is defined
4,
as:
number of reaction events
number of photons absorbed Suppose the quantum yield
for the reaction CH;X - CH3 + X is =0.24. A cuvette con-
taining a solution of CH3X is irradiated with 280-nm light with
a power of 885 mW for 10.0 minutes. Assuming total absorp-
tion of the light by the sample, what is the maximum amount
(in moles) of CH3X that breaks apart?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax