The rate constant for the second order reaction 2 NO2 - 2 NO + O2 is 0.24 M's. If a 1.38 M sample of NO2 reacts for 47.7 seconds, what concentration of NO2 wil remain? Report your answer to two decimal places. |Al = - kt +|Alo InJAl = - kt + InlAlo TAI = kt + TAlo
The rate constant for the second order reaction 2 NO2 - 2 NO + O2 is 0.24 M's. If a 1.38 M sample of NO2 reacts for 47.7 seconds, what concentration of NO2 wil remain? Report your answer to two decimal places. |Al = - kt +|Alo InJAl = - kt + InlAlo TAI = kt + TAlo
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 39QRT
Related questions
Question

Transcribed Image Text:The rate constant for the second order reaction 2 NO2 - 2 NO + O2 is 0.24 M's. If a 1.38 M sample of NO2 reacts for 47.7 seconds, what concentration of NO2 wil
remain?
Report your answer to two decimal places.
|Al = - kt +|Alo
InJAl = - kt + InlAlo
TAI
= kt +
TAlo
Expert Solution
Step 1
For a second order reaction, rate equation is given as : A0 = A + kt
Initial concentration (A0) = 1.38 M,
Rate constant (k) = 0.24 M-1 s-1
Time (t) = 47.7 s
Substiting the values in the integrated rate equation for 2nd order is we get,
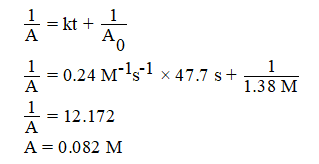
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning