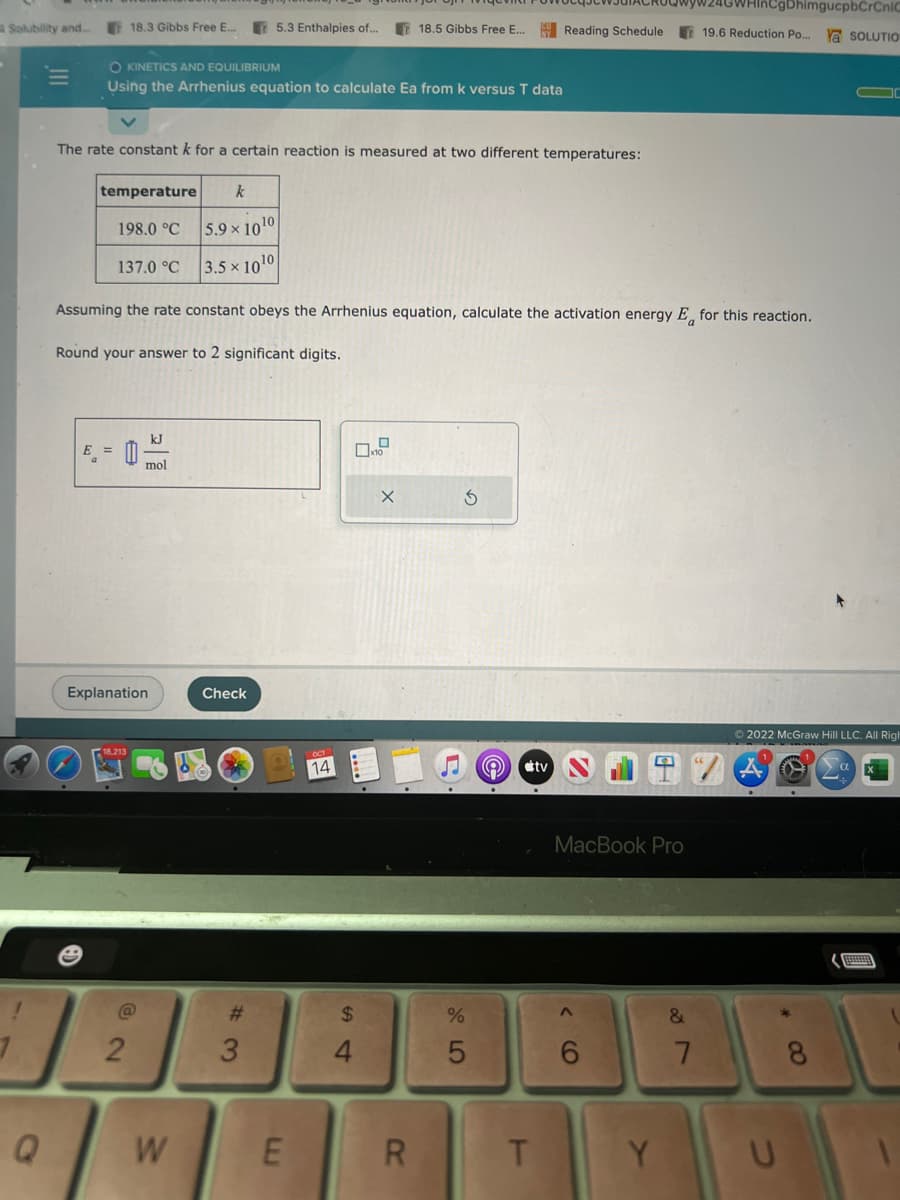
The rate constant k for a certain reaction is measured at two different temperatures: temperature k 5.9 x 1010 3.5 x 1010 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. Round your answer to 2 significant digits. 198.0 °C E = 137.0 °C mol ☐x10 X S
The rate constant k for a certain reaction is measured at two different temperatures: temperature k 5.9 x 1010 3.5 x 1010 Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction. Round your answer to 2 significant digits. 198.0 °C E = 137.0 °C mol ☐x10 X S
Chapter9: Aqueous Solutions And Chemical Equilibria
Section: Chapter Questions
Problem 9.8QAP
Related questions
Question
Transcribed Image Text:Solubility and... 18.3 Gibbs Free E...
Q
|||
OKINETICS AND EQUILIBRIUM
Using the Arrhenius equation to calculate Ea from k versus T data
temperature k
The rate constant k for a certain reaction is measured at two different temperatures:
E =
198.0 °C
137.0 °C
5.9 x 1010
3.5 x 1010
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy E for this reaction.
Round your answer to 2 significant digits.
kJ
mol
Explanation
@2
W
5.3 Enthalpies of...
Check
#
3
E
14
54
$
4
18.5 Gibbs Free E....
x10
X
R
%
5
Reading Schedule
© stv
T
MacBook Pro
^
6
Y
yw246WHInCgDhimgucpbCrCnic
19.6 Reduction Po... Y SOLUTIO
&
7
© 2022 McGraw Hill LLC. All Righ
U
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

